Graphene cover-promoted metal-catalyzed reactions
- PMID: 25404332
- PMCID: PMC4260550
- DOI: 10.1073/pnas.1416368111
Graphene cover-promoted metal-catalyzed reactions
Abstract
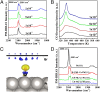
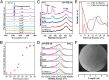
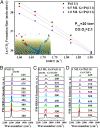
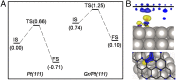
Graphitic overlayers on metals have commonly been considered as inhibitors for surface reactions due to their chemical inertness and physical blockage of surface active sites. In this work, however, we find that surface reactions, for instance, CO adsorption/desorption and CO oxidation, can take place on Pt(111) surface covered by monolayer graphene sheets. Surface science measurements combined with density functional calculations show that the graphene overlayer weakens the strong interaction between CO and Pt and, consequently, facilitates the CO oxidation with lower apparent activation energy. These results suggest that interfaces between graphitic overlayers and metal surfaces act as 2D confined nanoreactors, in which catalytic reactions are promoted. The finding contrasts with the conventional knowledge that graphitic carbon poisons a catalyst surface but opens up an avenue to enhance catalytic performance through coating of metal catalysts with controlled graphitic covers.
Keywords: CO oxidation; confinement effect; graphene; interface catalysis; platinum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schlögl R. Carbons. In: Ertl G, Knözinger H, Schüth F, Weitkamp J, editors. In Handbook of Heterogeneous Catalysis. Vol 1. Wiley-VCH; Weinheim, Germany: 2008. pp. 357–427.
-
- Goodman DW, Kelley RD, Madey TE, Yates JTJ. Kinetics of the hydrogenation of CO over a single crystal nickel catalyst. J Catal. 1980;63(1):226–234.
-
- Davis SM, Zaera F, Somorjai GA. The reactivity and composition of strongly adsorbed carbonaceous deposits on platinum. Model of the working hydrocarbon coversion catalyst. J Catal. 1982;77(2):439–459.
-
- Webb G. The formation and role of carbonaceous residues in metal-catalysed reactions of hydrocarbons. Catal Today. 1990;7(2):139–155.
-
- Brandt B, et al. Isomerization and hydrogenation of cis-2-butene on Pd model catalyst. J Phys Chem C. 2008;112(30):11408–11420.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

