OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence
- PMID: 25404365
- PMCID: PMC4272895
- DOI: 10.4049/jimmunol.1401644
OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence
Abstract
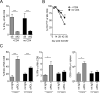
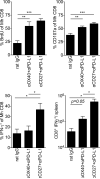
Exhaustion of chronically stimulated CD8(+) T cells is a significant obstacle to immune control of chronic infections or tumors. Although coinhibitory checkpoint blockade with anti-programmed death ligand 1 (PD-L1) Ab can restore functions to exhausted T cell populations, recovery is often incomplete and dependent upon the pool size of a quiescent T-bet(high) subset that expresses lower levels of PD-1. In a model in which unhelped, HY-specific CD8(+) T cells gradually lose function following transfer to male bone marrow transplantation recipients, we have explored the effect of shifting the balance away from coinhibition and toward costimulation by combining anti-PD-L1 with agonistic Abs to the TNFR superfamily members, OX40 and CD27. Several weeks following T cell transfer, both agonistic Abs, but especially anti-CD27, demonstrated synergy with anti-PD-L1 by enhancing CD8(+) T cell proliferation and effector cytokine generation. Anti-CD27 and anti-PD-L1 synergized by downregulating the expression of multiple quiescence-related genes concomitant with a reduced frequency of T-bet(high) cells within the exhausted population. However, in the presence of persistent Ag, the CD8(+) T cell response was not sustained and the overall size of the effector cytokine-producing pool eventually contracted to levels below that of controls. Thus, CD27-mediated costimulation can synergize with coinhibitory checkpoint blockade to switch off molecular programs for quiescence in exhausted T cell populations, but at the expense of losing precursor cells required to maintain a response.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



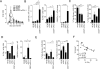
References
-
- Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. - PubMed
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

