Atenolol versus losartan in children and young adults with Marfan's syndrome
- PMID: 25405392
- PMCID: PMC4386623
- DOI: 10.1056/NEJMoa1404731
Atenolol versus losartan in children and young adults with Marfan's syndrome
Abstract
Background: Aortic-root dissection is the leading cause of death in Marfan's syndrome. Studies suggest that with regard to slowing aortic-root enlargement, losartan may be more effective than beta-blockers, the current standard therapy in most centers.
Methods: We conducted a randomized trial comparing losartan with atenolol in children and young adults with Marfan's syndrome. The primary outcome was the rate of aortic-root enlargement, expressed as the change in the maximum aortic-root-diameter z score indexed to body-surface area (hereafter, aortic-root z score) over a 3-year period. Secondary outcomes included the rate of change in the absolute diameter of the aortic root; the rate of change in aortic regurgitation; the time to aortic dissection, aortic-root surgery, or death; somatic growth; and the incidence of adverse events.
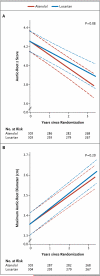
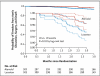
Results: From January 2007 through February 2011, a total of 21 clinical centers enrolled 608 participants, 6 months to 25 years of age (mean [±SD] age, 11.5±6.5 years in the atenolol group and 11.0±6.2 years in the losartan group), who had an aortic-root z score greater than 3.0. The baseline-adjusted rate of change in the mean (±SE) aortic-root z score did not differ significantly between the atenolol group and the losartan group (-0.139±0.013 and -0.107±0.013 standard-deviation units per year, respectively; P=0.08). Both slopes were significantly less than zero, indicating a decrease in the aortic-root diameter relative to body-surface area with either treatment. The 3-year rates of aortic-root surgery, aortic dissection, death, and a composite of these events did not differ significantly between the two treatment groups.
Conclusions: Among children and young adults with Marfan's syndrome who were randomly assigned to losartan or atenolol, we found no significant difference in the rate of aortic-root dilatation between the two treatment groups over a 3-year period. (Funded by the National Heart, Lung, and Blood Institute and others; ClinicalTrials.gov number, NCT00429364.).
Figures
Comment in
-
Of Marfan's syndrome, mice, and medications.N Engl J Med. 2014 Nov 27;371(22):2127-8. doi: 10.1056/NEJMe1412950. Epub 2014 Nov 18. N Engl J Med. 2014. PMID: 25405389 No abstract available.
-
Atenolol versus Losartan in Marfan's Syndrome.N Engl J Med. 2015 Mar 5;372(10):980-1. doi: 10.1056/NEJMc1500128. N Engl J Med. 2015. PMID: 25738680 No abstract available.
-
Atenolol versus Losartan in Marfan's Syndrome.N Engl J Med. 2015 Mar 5;372(10):977-8. doi: 10.1056/NEJMc1500128. N Engl J Med. 2015. PMID: 25738681 No abstract available.
-
Atenolol versus Losartan in Marfan's Syndrome.N Engl J Med. 2015 Mar 5;372(10):978-9. doi: 10.1056/NEJMc1500128. N Engl J Med. 2015. PMID: 25738682 No abstract available.
-
Atenolol versus Losartan in Marfan's Syndrome.N Engl J Med. 2015 Mar 5;372(10):980. doi: 10.1056/NEJMc1500128. N Engl J Med. 2015. PMID: 25738683 No abstract available.
-
[Drugs news].Arch Pediatr. 2015 Nov;22(11):1200-6. doi: 10.1016/j.arcped.2015.08.001. Epub 2015 Sep 15. Arch Pediatr. 2015. PMID: 26385645 French. No abstract available.
References
-
- Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–41. - PubMed
-
- Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005;111(11):e150–e157. - PubMed
-
- Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL109741/HL/NHLBI NIH HHS/United States
- U01 HL068269/HL/NHLBI NIH HHS/United States
- U01 HL068279/HL/NHLBI NIH HHS/United States
- U10 HL109743/HL/NHLBI NIH HHS/United States
- U01 HL068290/HL/NHLBI NIH HHS/United States
- U01 HL068288/HL/NHLBI NIH HHS/United States
- U10 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068281/HL/NHLBI NIH HHS/United States
- U01 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068292/HL/NHLBI NIH HHS/United States
- U10 HL109673/HL/NHLBI NIH HHS/United States
- U01 HL068285/HL/NHLBI NIH HHS/United States
- U01 HL085057/HL/NHLBI NIH HHS/United States
- U10 HL109778/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
