Activation of the NLRP3 inflammasome complex is not required for stress-induced death of pancreatic islets
- PMID: 25405767
- PMCID: PMC4236141
- DOI: 10.1371/journal.pone.0113128
Activation of the NLRP3 inflammasome complex is not required for stress-induced death of pancreatic islets
Abstract
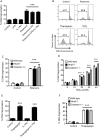
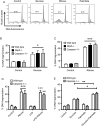
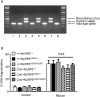
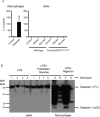
Loss of pancreatic beta cells is a feature of type-2 diabetes. High glucose concentrations induce endoplasmic reticulum (ER) and oxidative stress-mediated apoptosis of islet cells in vitro. ER stress, oxidative stress and high glucose concentrations may also activate the NLRP3 inflammasome leading to interleukin (IL)-1β production and caspase-1 dependent pyroptosis. However, whether IL-1β or intrinsic NLRP3 inflammasome activation contributes to beta cell death is controversial. This possibility was examined in mouse islets. Exposure of islets lacking functional NLRP3 or caspase-1 to H2O2, rotenone or thapsigargin induced similar cell death as in wild-type islets. This suggests that oxidative or ER stress do not cause inflammasome-mediated cell death. Similarly, deficiency of NLRP3 inflammasome components did not provide any protection from glucose, ribose or gluco-lipotoxicity. Finally, genetic activation of NLRP3 specifically in beta cells did not increase IL-1β production or cell death, even in response to glucolipotoxicity. Overall, our results show that glucose-, ER stress- or oxidative stress-induced cell death in islet cells is not dependent on intrinsic activation of the NLRP3 inflammasome.
Conflict of interest statement
Figures
References
-
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, et al. (2002) Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 45: 85–96. - PubMed
-
- Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, et al. (1988) Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9: 151–159. - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

