Task Dependence, Tissue Specificity, and Spatial Distribution of Widespread Activations in Large Single-Subject Functional MRI Datasets at 7T
- PMID: 25405938
- PMCID: PMC4635913
- DOI: 10.1093/cercor/bhu148
Task Dependence, Tissue Specificity, and Spatial Distribution of Widespread Activations in Large Single-Subject Functional MRI Datasets at 7T
Abstract
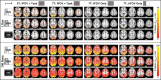
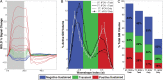
It was recently shown that when large amounts of task-based blood oxygen level-dependent (BOLD) data are combined to increase contrast- and temporal signal-to-noise ratios, the majority of the brain shows significant hemodynamic responses time-locked with the experimental paradigm. Here, we investigate the biological significance of such widespread activations. First, the relationship between activation extent and task demands was investigated by varying cognitive load across participants. Second, the tissue specificity of responses was probed using the better BOLD signal localization capabilities of a 7T scanner. Finally, the spatial distribution of 3 primary response types--namely positively sustained (pSUS), negatively sustained (nSUS), and transient--was evaluated using a newly defined voxel-wise waveshape index that permits separation of responses based on their temporal signature. About 86% of gray matter (GM) became significantly active when all data entered the analysis for the most complex task. Activation extent scaled with task load and largely followed the GM contour. The most common response type was nSUS BOLD, irrespective of the task. Our results suggest that widespread activations associated with extremely large single-subject functional magnetic resonance imaging datasets can provide valuable information about the functional organization of the brain that goes undetected in smaller sample sizes.
Keywords: activation extent; negative BOLD; single subject; transients.
Published by Oxford University Press 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures




References
-
- Cauda F, Costa T, Diano M, Sacco K, Duca S, Geminiani G, Torta DM. 2014. Massive modulation of brain areas after mechanical pain stimulation: a time-resolved fMRI study. Cereb Cortex. 24:2991–3005. - PubMed
-
- Cohen MS. 1997. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 6:93–103. - PubMed
-
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

