Antibiotic stewardship in the intensive care unit
- PMID: 25405992
- PMCID: PMC4281952
- DOI: 10.1186/s13054-014-0480-6
Antibiotic stewardship in the intensive care unit
Abstract
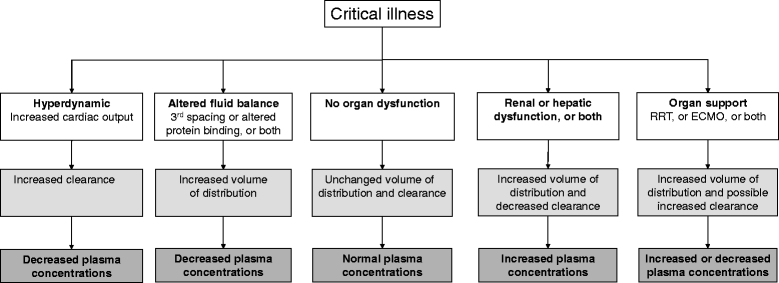
The rapid emergence and dissemination of antimicrobial-resistant microorganisms in ICUs worldwide constitute a problem of crisis dimensions. The root causes of this problem are multifactorial, but the core issues are clear. The emergence of antibiotic resistance is highly correlated with selective pressure resulting from inappropriate use of these drugs. Appropriate antibiotic stewardship in ICUs includes not only rapid identification and optimal treatment of bacterial infections in these critically ill patients, based on pharmacokinetic-pharmacodynamic characteristics, but also improving our ability to avoid administering unnecessary broad-spectrum antibiotics, shortening the duration of their administration, and reducing the numbers of patients receiving undue antibiotic therapy. Either we will be able to implement such a policy or we and our patients will face an uncontrollable surge of very difficult-to-treat pathogens.
Figures


References
-
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. Antibiotic resistance - the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. - DOI - PubMed
-
- Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–1083. doi: 10.1093/cid/ciu027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

