Low temperature-induced circulating triiodothyronine accelerates seasonal testicular regression
- PMID: 25406020
- PMCID: PMC4298317
- DOI: 10.1210/en.2014-1741
Low temperature-induced circulating triiodothyronine accelerates seasonal testicular regression
Abstract
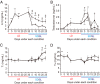
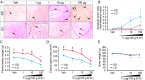
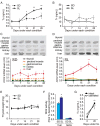
In temperate zones, animals restrict breeding to specific seasons to maximize the survival of their offspring. Birds have evolved highly sophisticated mechanisms of seasonal regulation, and their testicular mass can change 100-fold within a few weeks. Recent studies on Japanese quail revealed that seasonal gonadal development is regulated by central thyroid hormone activation within the hypothalamus, depending on the photoperiodic changes. By contrast, the mechanisms underlying seasonal testicular regression remain unclear. Here we show the effects of short day and low temperature on testicular regression in quail. Low temperature stimulus accelerated short day-induced testicular regression by shutting down the hypothalamus-pituitary-gonadal axis and inducing meiotic arrest and germ cell apoptosis. Induction of T3 coincided with the climax of testicular regression. Temporal gene expression analysis over the course of apoptosis revealed the suppression of LH response genes and activation of T3 response genes involved in amphibian metamorphosis within the testis. Daily ip administration of T3 mimicked the effects of low temperature stimulus on germ cell apoptosis and testicular mass. Although type 2 deiodinase, a thyroid hormone-activating enzyme, in the brown adipose tissue generates circulating T3 under low-temperature conditions in mammals, there is no distinct brown adipose tissue in birds. In birds, type 2 deiodinase is induced by low temperature exclusively in the liver, which appears to be caused by increased food consumption. We conclude that birds use low temperature-induced circulating T3 not only for adaptive thermoregulation but also to trigger apoptosis to accelerate seasonal testicular regression.
Figures





References
-
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythm. 2001;16:365–380. - PubMed
-
- Nakane Y, Shimmura T, Abe H, Yoshimura T. Intrinsic photosensitivity of a deep brain photoreceptor. Curr Biol. 2014;24:R596–R597. - PubMed
-
- Nakao N, Ono H, Yamamura T, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

