Role of nuclear progesterone receptor isoforms in uterine pathophysiology
- PMID: 25406186
- PMCID: PMC4366574
- DOI: 10.1093/humupd/dmu056
Role of nuclear progesterone receptor isoforms in uterine pathophysiology
Abstract
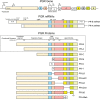
Background: Progesterone is a key hormonal regulator of the female reproductive system. It plays a major role to prepare the uterus for implantation and in the establishment and maintenance of pregnancy. Actions of progesterone on the uterine tissues (endometrium, myometrium and cervix) are mediated by the combined effects of two progesterone receptor (PR) isoforms, designated PR-A and PR-B. Both receptors function primarily as ligand-activated transcription factors. Progesterone action on the uterine tissues is qualitatively and quantitatively determined by the relative levels and transcriptional activities of PR-A and PR-B. The transcriptional activity of the PR isoforms is affected by specific transcriptional coregulators and by PR post-translational modifications that affect gene promoter targeting. In this context, appropriate temporal and cell-specific expression and function of PR-A and PR-B are critical for normal uterine function.
Methods: Relevant studies describing the role of PRs in uterine physiology and pathology (endometriosis, uterine leiomyoma, endometrial cancer, cervical cancer and recurrent pregnancy loss) were comprehensively searched using PubMed, Cochrane Library, Web of Science, and Google Scholar and critically reviewed.
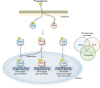
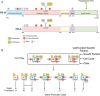
Results: Progesterone, acting through PR-A and PR-B, regulates the development and function of the endometrium and induces changes in cells essential for implantation and the establishment and maintenance of pregnancy. During pregnancy, progesterone via the PRs promotes myometrial relaxation and cervical closure. Withdrawal of PR-mediated progesterone signaling triggers menstruation and parturition. PR-mediated progesterone signaling is anti-mitogenic in endometrial epithelial cells, and as such, mitigates the tropic effects of estrogen on eutopic normal endometrium, and on ectopic implants in endometriosis. Similarly, ligand-activated PRs function as tumor suppressors in endometrial cancer cells through inhibition of key cellular signaling pathways required for growth. In contrast, progesterone via PR activation appears to increase leiomyoma growth. The exact role of PRs in cervical cancer is unclear. PRs regulate implantation and therefore aberrant PR function may be implicated in recurrent pregnancy loss (RPL). PRs likely regulate key immunogenic factors involved in RPL. However, the exact role of PRs in the pathophysiology of RPL and the use of progesterone for therapeutic benefit remains uncertain.
Conclusions: PRs are key mediators of progesterone action in uterine tissues and are essential for normal uterine function. Aberrant PR function (due to abnormal expression and/or function) is a major cause of uterine pathophysiology. Further investigation of the underlying mechanisms of PR isoform action in the uterus is required, as this knowledge will afford the opportunity to create progestin/PR-based therapeutics to treat various uterine pathologies.
Keywords: progesterone; progesterone receptors; uterine pathophysiology.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

