Modulation of VEGF-induced retinal vascular permeability by peroxisome proliferator-activated receptor-β/δ
- PMID: 25406289
- PMCID: PMC4271638
- DOI: 10.1167/iovs.14-14217
Modulation of VEGF-induced retinal vascular permeability by peroxisome proliferator-activated receptor-β/δ
Abstract
Purpose: Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability contributes to diabetic macular edema (DME), a serious vision-threatening condition. Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) antagonist/reverse agonist, GSK0660, inhibits VEGF-induced human retinal microvascular endothelial cell (HRMEC) proliferation, tubulogenesis, and oxygen-induced retinal vasculopathy in newborn rats. These VEGF-induced HRMEC behaviors and VEGF-induced disruption of endothelial cell junctional complexes may well share molecular signaling events. Thus, we sought to examine the role of PPARβ/δ in VEGF-induced retinal hyperpermeability.
Methods: Transendothelial electrical resistance (TEER) measurements were performed on HRMEC monolayers to assess permeability. Claudin-1/Claudin-5 localization in HRMEC monolayers was determined by immunocytochemistry. Extracellular signal-regulated protein kinases 1 and 2 (Erk 1/2) phosphorylation, VEGF receptor 1 (VEGFR1) and R2 were assayed by Western blot analysis. Expression of VEGFR1 and R2 was measured by quantitative RT-PCR. Last, retinal vascular permeability was assayed in vivo by Evans blue extravasation.
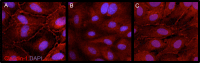
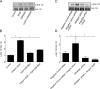
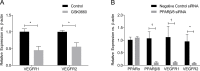
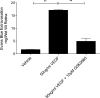
Results: Human retinal microvascular endothelial cell monolayers treated with VEGF for 24 hours showed decreased TEER values that were completely reversed by the highest concentration of GSK0660 (10 μM) and PPARβ/δ-directed siRNA (20 μM). In HRMEC treated with VEGF, GSK0660 stabilized tight-junctions as evidenced by Claudin-1 staining, reduced phosphorylation of Erk1/2, and reduced VEGFR1/2 expression. Peroxisome proliferator-activated receptor β/δ siRNA had a similar effect on VEGFR expression and Claudin-1, supporting the specificity of GSK0660 in our experiments. Last, GSK0660 significantly inhibited VEGF-induced retinal vascular permeability and reduced retinal VEGFR1and R2 levels in C57BL/6 mice.
Conclusions: These data suggest a protective effect for PPARβ/δ antagonism against VEGF-induced vascular permeability, possibly through reduced VEGFR expression. Therefore, antagonism/reverse agonism of PPARβ/δ siRNA may represent a novel therapeutic methodology against retinal hyperpermeability and is worthy of future investigation.
Keywords: vascular permeability, PPARβ; δ, VEGF.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures


References
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. - PubMed
-
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995; 102: 7–16. - PubMed
-
- Klein R, Klein BE, Moss SE, Linton KL. The Beaver Dam Eye Study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology. 1992; 99: 58–62. - PubMed
-
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007; 298: 902–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

