Whirlin and PDZ domain-containing 7 (PDZD7) proteins are both required to form the quaternary protein complex associated with Usher syndrome type 2
- PMID: 25406310
- PMCID: PMC4276872
- DOI: 10.1074/jbc.M114.610535
Whirlin and PDZ domain-containing 7 (PDZD7) proteins are both required to form the quaternary protein complex associated with Usher syndrome type 2
Abstract
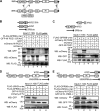
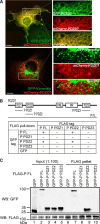
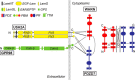
Usher syndrome (USH) is the leading genetic cause of combined hearing and vision loss. Among the three USH clinical types, type 2 (USH2) occurs most commonly. USH2A, GPR98, and WHRN are three known causative genes of USH2, whereas PDZD7 is a modifier gene found in USH2 patients. The proteins encoded by these four USH genes have been proposed to form a multiprotein complex, the USH2 complex, due to interactions found among some of these proteins in vitro, their colocalization in vivo, and mutual dependence of some of these proteins for their normal in vivo localizations. However, evidence showing the formation of the USH2 complex is missing, and details on how this complex is formed remain elusive. Here, we systematically investigated interactions among the intracellular regions of the four USH proteins using colocalization, yeast two-hybrid, and pull-down assays. We show that multiple domains of the four USH proteins interact among one another. Importantly, both WHRN and PDZD7 are required for the complex formation with USH2A and GPR98. In this USH2 quaternary complex, WHRN prefers to bind to USH2A, whereas PDZD7 prefers to bind to GPR98. Interaction between WHRN and PDZD7 is the bridge between USH2A and GPR98. Additionally, the USH2 quaternary complex has a variable stoichiometry. These findings suggest that a non-obligate, short term, and dynamic USH2 quaternary protein complex may exist in vivo. Our work provides valuable insight into the physiological role of the USH2 complex in vivo and informs possible reconstruction of the USH2 complex for future therapy.
Keywords: GPR98; Hair Cell; PDZ Domain; PDZD7; Photoreceptor; Protein Complex; Protein-Protein Interaction; USH2A; Usher Syndrome; WHRN.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures











References
-
- Ebermann I., Phillips J. B., Liebau M. C., Koenekoop R. K., Schermer B., Lopez I., Schäfer E., Roux A. F., Dafinger C., Bernd A., Zrenner E., Claustres M., Blanco B., Nürnberg G., Nürnberg P., Ruland R., Westerfield M., Benzing T., Bolz H. J. (2010) PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J. Clin. Invest. 120, 1812–1823 - PMC - PubMed
-
- Ebermann I., Scholl H. P., Charbel Issa P., Becirovic E., Lamprecht J., Jurklies B., Millán J. M., Aller E., Mitter D., Bolz H. (2007) A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum. Genet. 121, 203–211 - PubMed
-
- Eudy J. D., Weston M. D., Yao S., Hoover D. M., Rehm H. L., Ma-Edmonds M., Yan D., Ahmad I., Cheng J. J., Ayuso C., Cremers C., Davenport S., Moller C., Talmadge C. B., Beisel K. W., Tamayo M., Morton C. C., Swaroop A., Kimberling W. J., Sumegi J. (1998) Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 280, 1753–1757 - PubMed
-
- Schneider E., Märker T., Daser A., Frey-Mahn G., Beyer V., Farcas R., Schneider-Ratzke B., Kohlschmidt N., Grossmann B., Bauss K., Napiontek U., Keilmann A., Bartsch O., Zechner U., Wolfrum U., Haaf T. (2009) Homozygous disruption of PDZD7 by reciprocal translocation in a consanguineous family: a new member of the Usher syndrome protein interactome causing congenital hearing impairment. Hum. Mol. Genet. 18, 655–666 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

