Measuring glutathione redox potential of HIV-1-infected macrophages
- PMID: 25406321
- PMCID: PMC4294471
- DOI: 10.1074/jbc.M114.588913
Measuring glutathione redox potential of HIV-1-infected macrophages
Abstract
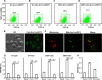
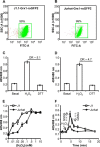
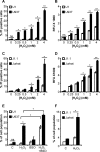
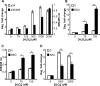
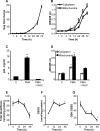
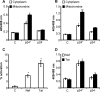
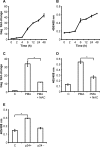
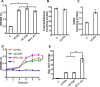
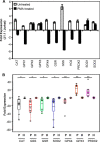
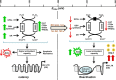
Redox signaling plays a crucial role in the pathogenesis of human immunodeficiency virus type-1 (HIV-1). The majority of HIV redox research relies on measuring redox stress using invasive technologies, which are unreliable and do not provide information about the contributions of subcellular compartments. A major technological leap emerges from the development of genetically encoded redox-sensitive green fluorescent proteins (roGFPs), which provide sensitive and compartment-specific insights into redox homeostasis. Here, we exploited a roGFP-based specific bioprobe of glutathione redox potential (E(GSH); Grx1-roGFP2) and measured subcellular changes in E(GSH) during various phases of HIV-1 infection using U1 monocytic cells (latently infected U937 cells with HIV-1). We show that although U937 and U1 cells demonstrate significantly reduced cytosolic and mitochondrial E(GSH) (approximately -310 mV), active viral replication induces substantial oxidative stress (E(GSH) more than -240 mV). Furthermore, exposure to a physiologically relevant oxidant, hydrogen peroxide (H2O2), induces significant deviations in subcellular E(GSH) between U937 and U1, which distinctly modulates susceptibility to apoptosis. Using Grx1-roGFP2, we demonstrate that a marginal increase of about ∼25 mV in E(GSH) is sufficient to switch HIV-1 from latency to reactivation, raising the possibility of purging HIV-1 by redox modulators without triggering detrimental changes in cellular physiology. Importantly, we show that bioactive lipids synthesized by clinical drug-resistant isolates of Mycobacterium tuberculosis reactivate HIV-1 through modulation of intracellular E(GSH). Finally, the expression analysis of U1 and patient peripheral blood mononuclear cells demonstrated a major recalibration of cellular redox homeostatic pathways during persistence and active replication of HIV.
Keywords: AIDS; Human Immunodeficiency Virus (HIV); Mycobacterium tuberculosis; Pathogenesis; Redox Signaling.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Perl A., Banki K. (2000) Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid. Redox Signal. 2, 551–573 - PubMed
-
- Pyo C. W., Yang Y. L., Yoo N. K., Choi S. Y. (2008) Reactive oxygen species activate HIV long terminal repeat via post-translational control of NF-κB. Biochem. Biophys. Res. Commun. 376, 180–185 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

