Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type
- PMID: 25406334
- PMCID: PMC4407759
- DOI: 10.1093/infdis/jiu647
Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type
Abstract
Background: During the 2012-2013 influenza season, there was cocirculation of influenza A(H3N2) and 2 influenza B lineage viruses in the United States.
Methods: Patients with acute cough illness for ≤7 days were prospectively enrolled and had swab samples obtained at outpatient clinics in 5 states. Influenza vaccination dates were confirmed by medical records. The vaccine effectiveness (VE) was estimated as [100% × (1 - adjusted odds ratio)] for vaccination in cases versus test-negative controls.
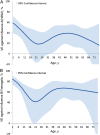
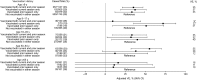
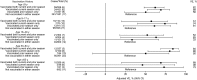
Results: Influenza was detected in 2307 of 6452 patients (36%); 1292 (56%) had influenza A(H3N2), 582 (25%) had influenza B/Yamagata, and 303 (13%) had influenza B/Victoria. VE was 49% (95% confidence interval [CI], 43%-55%) overall, 39% (95% CI, 29%-47%) against influenza A(H3N2), 66% (95% CI, 58%-73%) against influenza B/Yamagata (vaccine lineage), and 51% (95% CI, 36%-63%) against influenza B/Victoria. VE against influenza A(H3N2) was highest among persons aged 50-64 years (52%; 95% CI, 33%-65%) and persons aged 6 months-8 years (51%; 95% CI, 32%-64%) and lowest among persons aged ≥65 years (11%; 95% CI, -41% to 43%). In younger age groups, there was evidence of residual protection from receipt of the 2011-2012 vaccine 1 year earlier.
Conclusions: The 2012-2013 vaccines were moderately effective in most age groups. Cross-lineage protection and residual effects from prior vaccination were observed and warrant further investigation.
Keywords: influenza; medically attended influenza; vaccine effectiveness.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures
References
-
- Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 2009; 199:159–67. - PubMed
-
- Belongia EA, Kieke BA, Donahue JG, et al. Influenza vaccine effectiveness in Wisconsin during the 2007–08 season: comparison of interim and final results. Vaccine 2011; 29:6558–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

