Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain
- PMID: 25406396
- PMCID: PMC4302931
- DOI: 10.1242/dev.107193
Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain
Abstract
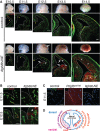
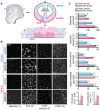
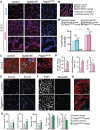
Vascular development of the central nervous system and blood-brain barrier (BBB) induction are closely linked processes. The role of factors that promote endothelial sprouting and vascular leak, such as vascular endothelial growth factor A, are well described, but the factors that suppress angiogenic sprouting and their impact on the BBB are poorly understood. Here, we show that integrin αVβ8 activates angiosuppressive TGFβ gradients in the brain, which inhibit endothelial cell sprouting. Loss of αVβ8 in the brain or downstream TGFβ1-TGFBR2-ALK5-Smad3 signaling in endothelial cells increases vascular sprouting, branching and proliferation, leading to vascular dysplasia and hemorrhage. Importantly, BBB function in Itgb8 mutants is intact during early stages of vascular dysgenesis before hemorrhage. By contrast, Pdgfb(ret/ret) mice, which exhibit severe BBB disruption and vascular leak due to pericyte deficiency, have comparatively normal vascular morphogenesis and do not exhibit brain hemorrhage. Our data therefore suggest that abnormal vascular sprouting and patterning, not BBB dysfunction, underlie developmental cerebral hemorrhage.
Keywords: Angiogenesis; Brain; CNS; Hemorrhage; Integrin αVβ8; Mouse; Sprouting; TGFβ.
© 2014. Published by The Company of Biologists Ltd.
Figures


References
-
- Anderson K. D., Pan L., Yang X.-M., Hughes V. C., Walls J. R., Dominguez M. G., Simmons M. V., Burfeind P., Xue Y., Wei Y. et al. (2011). Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 108, 2807-2812 10.1073/pnas.1019761108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01 HL078564/HL/NHLBI NIH HHS/United States
- R01 GM060514/GM/NIGMS NIH HHS/United States
- R37 HL053949/HL/NHLBI NIH HHS/United States
- K99-R00 NS070920/NS/NINDS NIH HHS/United States
- K12HD047349/HD/NICHD NIH HHS/United States
- R00 NS070920/NS/NINDS NIH HHS/United States
- K12 HD047349/HD/NICHD NIH HHS/United States
- R37 HL53949/HL/NHLBI NIH HHS/United States
- R01 NS19090/NS/NINDS NIH HHS/United States
- Z01 HL005702/ImNIH/Intramural NIH HHS/United States
- R01 HL078564/HL/NHLBI NIH HHS/United States
- R01 NS019090/NS/NINDS NIH HHS/United States
- 5R01 GM060514/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

