A nuclear chocolate box: the periodic table of nuclear medicine
- PMID: 25406520
- PMCID: PMC6205633
- DOI: 10.1039/c4dt02846e
A nuclear chocolate box: the periodic table of nuclear medicine
Abstract
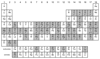
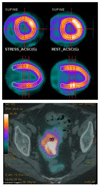
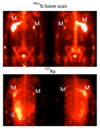
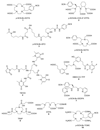
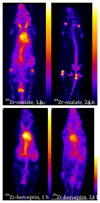
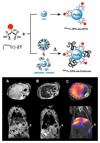
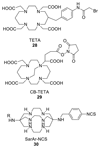
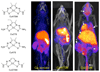
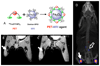
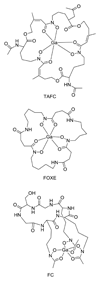
Radioisotopes of elements from all parts of the periodic table find both clinical and research applications in radionuclide molecular imaging and therapy (nuclear medicine). This article provides an overview of these applications in relation to both the radiological properties of the radionuclides and the chemical properties of the elements, indicating past successes, current applications and future opportunities and challenges for inorganic chemistry.
Figures
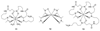





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

