3D GRASE pulsed arterial spin labeling at multiple inflow times in patients with long arterial transit times: comparison with dynamic susceptibility-weighted contrast-enhanced MRI at 3 Tesla
- PMID: 25407272
- PMCID: PMC4348376
- DOI: 10.1038/jcbfm.2014.200
3D GRASE pulsed arterial spin labeling at multiple inflow times in patients with long arterial transit times: comparison with dynamic susceptibility-weighted contrast-enhanced MRI at 3 Tesla
Abstract
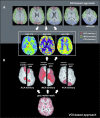
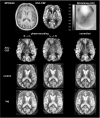
Pulsed arterial spin labeling (PASL) at multiple inflow times (multi-TIs) is advantageous for the measurement of brain perfusion in patients with long arterial transit times (ATTs) as in steno-occlusive disease, because bolus-arrival-time can be measured and blood flow measurements can be corrected accordingly. Owing to its increased signal-to-noise ratio, a combination with a three-dimensional gradient and spin echo (GRASE) readout allows acquiring a sufficient number of multi-TIs within a clinically feasible acquisition time of 5 minutes. We compared this technique with the clinical standard dynamic susceptibility-weighted contrast-enhanced imaging-magnetic resonance imaging in patients with unilateral stenosis >70% of the internal carotid or middle cerebral artery (MCA) at 3 Tesla. We performed qualitative (assessment by three expert raters) and quantitative (region of interest (ROI)/volume of interest (VOI) based) comparisons. In 43 patients, multi-TI PASL-GRASE showed perfusion alterations with moderate accuracy in the qualitative analysis. Quantitatively, moderate correlation coefficients were found for the MCA territory (ROI based: r=0.52, VOI based: r=0.48). In the anterior cerebral artery (ACA) territory, a readout related right-sided susceptibility artifact impaired correlation (ROI based: r=0.29, VOI based: r=0.34). Arterial transit delay artifacts were found only in 12% of patients. In conclusion, multi-TI PASL-GRASE can correct for arterial transit delay in patients with long ATTs. These results are promising for the transfer of ASL to the clinical practice.
Figures


References
-
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med 1992; 23: 37–45. - PubMed
-
- Wintermark M, Sesay M, Barbier E, Borbély K, Dillon WP, Eastwood JD et al. Comparative overview of brain perfusion imaging techniques. Stroke 2005; 36: e83–e99. - PubMed
-
- Pizzini FB, Farace P, Manganotti P, Zoccatelli G, Bongiovanni LG, Golay X et al. Cerebral perfusion alterations in epileptic patients during peri-ictal and post-ictal phase: PASL vs DSC-MRI. Magn Reson Imaging 2013; 31: 1001–1005. - PubMed
-
- White CM, Pope WB, Zaw T, Qiao J, Naeini KM, Lai A et al. Regional and voxel-wise comparisons of blood flow measurements between dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) and arterial spin labeling (ASL) in brain tumors. JNeuroimaging 2014; 24: 23–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

