Antiproliferative effect of the jararhagin toxin on B16F10 murine melanoma
- PMID: 25407317
- PMCID: PMC4289281
- DOI: 10.1186/1472-6882-14-446
Antiproliferative effect of the jararhagin toxin on B16F10 murine melanoma
Abstract
Background: Malignant melanoma is a less common but highly dangerous form of skin cancer; it starts in the melanocytes cells found in the outer layer of the skin. Jararhagin toxin, a metalloproteinase isolated from Bothrops jararaca snake venom acts upon several biological processes, as inflammation, pain, platelet aggregation, proliferation and apoptosis, though not yet approved for use, may one day be employed to treat tumors.
Methods: B16F10 murine melanoma cells were treated with jararhagin (jara), a disintegrin-like metalloproteinase isolated from Bothrops jararaca snake venom, and jari (catalytic domain inactivated with 1,10-phenanthroline). Viability and adhesion cells were evaluated by MTT assay. The expression of caspase-3 active, phases of the cell cycle and apoptosis were assessed by flow cytometry. We analyze in vivo the effects of jararhagin on melanoma growth, apoptosis and metastasis.
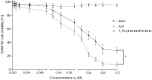
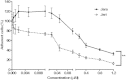
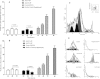
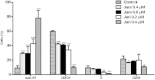
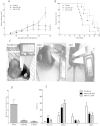
Results: The tumor cells acquired round shapes, lost cytoplasmic expansions, formed clusters in suspension and decreased viability. Jari was almost 20 times more potent toxin than jara based on IC50 values and on morphological changes of the cells, also observed by scanning electron microscopy. Flow cytometry analysis showed 48.3% decrease in the proliferation rate of cells and 47.2% increase in apoptosis (jara) and necrosis (jari), following 1.2 μM jara and 0.1 μM jari treatments. Caspase-3 activity was increased whereas G0/G1 cell cycle phase was on the decline. Proliferative rate was assessed by staining with 5,6-carboxyfluoresceindiacetate succinimidyl ester, showing a significant decrease in proliferation at all concentrations of both toxins.
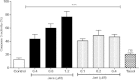
Conclusions: In vivo treatment of the toxins was observed reduction in the incidence of nodules, and metastasis and antiproliferative inhibition capacity. This data strengthens the potential use jararhagin as an anti-neoplastic drug.
Figures


References
-
- Beviglia L, Stewart GJ, Niewiarowski S. Effect of four disintegrins on the adhesive and metastatic properties of B16F10 melanoma cells in a murine model. Oncogene Res. 1995;7:7–20. - PubMed
-
- Danen EHJ, Marcinkiewicz C, Cornelissen IMHA, Van Kraats AA, Pachter JA, Ruiter DJ, Niewiarowski S, Van Muijen GNP. The disintegrin eristostatin interferes with integrin α4β1 function and with experimental metastasis of human melanoma cells. Exp Cell Res. 1998;238:188–196. doi: 10.1006/excr.1997.3821. - DOI - PubMed
-
- Staiano N, Garbi C, Squillacioti C, Espósito S, Di Martino E, Belisario MA, Nitsch L, Di Natale P. Echistatin induces decrease of pp125FAK phosphorilation, disassembly of actin cytoskeleton and focal adhesions, and detachment of fibronectin-adherent melanoma cells. Eur J Cell Biol. 1997;73:298–305. - PubMed
-
- Della Morte R, Squillacioti C, Garbi C, Derkinderen P, Maria A, Belisario MA, Girault JA, Natale PD, Nitsch L, Staiano N. Echistatin inhibits pp125FAK autophosphorylation, paxillin phosphorylation and pp125FAK±paxillin interaction in fibronectin-adherent melanoma cells. Eur J Biochem. 2000;267:5047–5054. doi: 10.1046/j.1432-1327.2000.01561.x. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1472-6882/14/446/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

