The stability of G6PD is affected by mutations with different clinical phenotypes
- PMID: 25407525
- PMCID: PMC4264219
- DOI: 10.3390/ijms151121179
The stability of G6PD is affected by mutations with different clinical phenotypes
Abstract
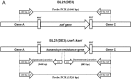
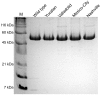
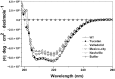
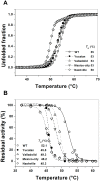
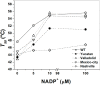
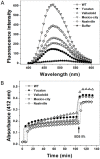
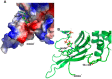
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzyme deficiency worldwide, causing a wide spectrum of conditions with severity classified from the mildest (Class IV) to the most severe (Class I). To correlate mutation sites in the G6PD with the resulting phenotypes, we studied four naturally occurring G6PD variants: Yucatan, Nashville, Valladolid and Mexico City. For this purpose, we developed a successful over-expression method that constitutes an easier and more precise method for obtaining and characterizing these enzymes. The k(cat) (catalytic constant) of all the studied variants was lower than in the wild-type. The structural rigidity might be the cause and the most evident consequence of the mutations is their impact on protein stability and folding, as can be observed from the protein yield, the T50 (temperature where 50% of its original activity is retained) values, and differences on hydrophobic regions. The mutations corresponding to more severe phenotypes are related to the structural NADP+ region. This was clearly observed for the Classes III and II variants, which became more thermostable with increasing NADP+, whereas the Class I variants remained thermolabile. The mutations produce repulsive electric charges that, in the case of the Yucatan variant, promote increased disorder of the C-terminus and consequently affect the binding of NADP+, leading to enzyme instability.
Figures

References
-
- Luzzatto L., Mehta A., Vulliamy T.J. Glucose-6-phosphate dehydrogenase deficiency. In: Scriver C.R., Beaudet A.L., et al., editors. Metabolic and Molecular Bases of Inherited Disease. 8th ed. Volume 179. McGraw-Hill; New York, NY, USA: 2001. pp. 4517–4553.
-
- Gaetani G.F., Galiano S., Canepa L., Ferraris A.M., Kirkman H.N. Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood. 1989;73:334–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

