Human semen contains exosomes with potent anti-HIV-1 activity
- PMID: 25407601
- PMCID: PMC4245725
- DOI: 10.1186/s12977-014-0102-z
Human semen contains exosomes with potent anti-HIV-1 activity
Abstract
Background: Exosomes are membranous nanovesicles secreted into the extracellular milieu by diverse cell types. Exosomes facilitate intercellular communication, modulate cellular pheno/genotype, and regulate microbial pathogenesis. Although human semen contains exosomes, their role in regulating infection with viruses that are sexually transmitted remains unknown. In this study, we used semen exosomes purified from healthy human donors to evaluate the role of exosomes on the infectivity of different strains of HIV-1 in a variety of cell lines.
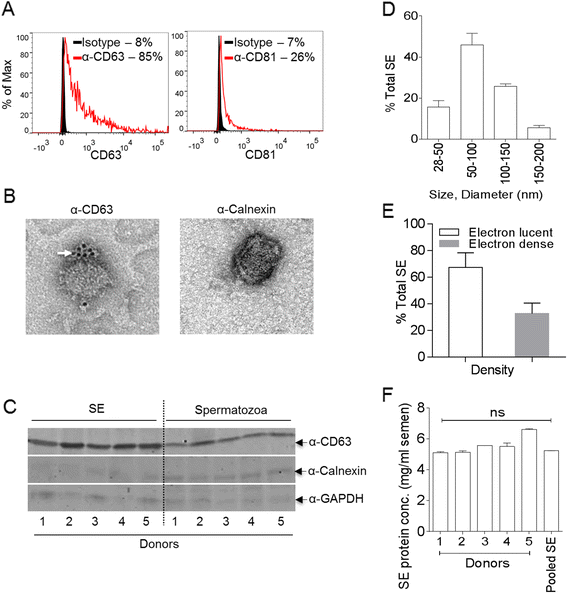
Results: We show that human semen contains a heterologous population of exosomes, enriched in mRNA encoding tetraspanin exosomal markers and various antiviral factors. Semen exosomes are internalized by recipient cells and upon internalization, inhibit replication of a broad array of HIV-1 strains. Remarkably, the anti-HIV-1 activity of semen exosomes is specific to retroviruses because semen exosomes blocked replication of the murine AIDS (mAIDS) virus complex (LP-BM5). However, exosomes from blood had no effect on HIV-1 or LP-BM5 replication. Additionally, semen and blood exosomes had no effect on replication of herpes simplex virus; types 1 and 2 (HSV1 and HSV2). Mechanistic studies indicate that semen exosomes exert a post-entry block on HIV-1 replication by orchestrating deleterious effects on particle-associated reverse transcriptase activity and infectivity.
Conclusions: These illuminating findings i) improve our knowledge of the cargo of semen exosomes, ii) reveal that semen exosomes possess anti-retroviral activity, and iii) suggest that semen exosome-mediated inhibition of HIV-1 replication may provide novel opportunities for the development of new therapeutics for HIV-1.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

