Effects of the dual peroxisome proliferator-activated receptor-α/γ agonist aleglitazar on renal function in patients with stage 3 chronic kidney disease and type 2 diabetes: a Phase IIb, randomized study
- PMID: 25407798
- PMCID: PMC4364102
- DOI: 10.1186/1471-2369-15-180
Effects of the dual peroxisome proliferator-activated receptor-α/γ agonist aleglitazar on renal function in patients with stage 3 chronic kidney disease and type 2 diabetes: a Phase IIb, randomized study
Abstract
Background: Type 2 diabetes is a major risk factor for chronic kidney disease, which substantially increases the risk of cardiovascular disease mortality. This Phase IIb safety study (AleNephro) in patients with stage 3 chronic kidney disease and type 2 diabetes, evaluated the renal effects of aleglitazar, a balanced peroxisome proliferator-activated receptor-α/γ agonist.
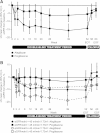
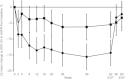
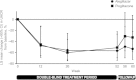
Methods: Patients were randomized to 52 weeks' double-blind treatment with aleglitazar 150 μg/day (n=150) or pioglitazone 45 mg/day (n=152), followed by an 8-week off-treatment period. The primary endpoint was non-inferiority for the difference between aleglitazar and pioglitazone in percentage change in estimated glomerular filtration rate from baseline to end of follow-up. Secondary endpoints included change from baseline in estimated glomerular filtration rate and lipid profiles at end of treatment.
Results: Mean estimated glomerular filtration rate change from baseline to end of follow-up was -2.7% (95% confidence interval: -7.7, 2.4) with aleglitazar versus -3.4% (95% confidence interval: -8.5, 1.7) with pioglitazone, establishing non-inferiority (0.77%; 95% confidence interval: -4.5, 6.0). Aleglitazar was associated with a 15% decrease in estimated glomerular filtration rate versus 5.4% with pioglitazone at end of treatment, which plateaued to 8 weeks and was not progressive. Superior improvements in high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides, with similar effects on glycosylated hemoglobin were observed with aleglitazar versus pioglitazone. No major safety concerns were identified.
Conclusions: The primary endpoint in AleNephro was met, indicating that in stage 3 chronic kidney disease patients with type 2 diabetes, the decrease in estimated glomerular filtration rate after 52 weeks' treatment with aleglitazar followed by 8 weeks off-treatment was reversible and comparable (non-inferior) to pioglitazone.
Trial registration: NCT01043029 January 5, 2010.
Figures
References
-
- Centers of Disease Control and Prevention . National Diabetes Statistics Report. 2014.
-
- Leiter LA, Fitchett DH, Gilbert RE, Gupta M, Mancini GB, McFarlane PA, Ross R, Teoh H, Verma S, Anand S, Camelon K, Chow CM, Cox JL, Després JP, Genest J, Harris SB, Lau DC, Lewanczuk R, Liu PP, Lonn EM, McPherson R, Poirier P, Qaadri S, Rabasa-Lhoret R, Rabkin SW, Sharma AM, Steele AW, Stone JA, Tardif JC, Tobe S, et al. Identification and management of cardiometabolic risk in Canada: a position paper by the cardiometabolic risk working group (executive summary) Can J Cardiol. 2011;27:124–131. doi: 10.1016/j.cjca.2011.01.016. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2369/15/180/prepub
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

