Circulating tumour cells in patients with urothelial tumours: Enrichment and in vitro culture
- PMID: 25408812
- PMCID: PMC4216304
- DOI: 10.5489/cuaj.1978
Circulating tumour cells in patients with urothelial tumours: Enrichment and in vitro culture
Abstract
Introduction: Results of clinical trials have demonstrated that circulating tumour cells (CTCs) are frequently detected in patients with urothelial tumours. The monitoring of CTCs has the potential to improve therapeutic management at an early stage and also to identify patients with increased risk of tumour progression or recurrence before the onset of clinically detected metastasis. In this study, we report a new effectively simplified methodology for a separation and in vitro culturing of viable CTCs from peripheral blood.
Method: We include patients diagnosed with 3 types of urothelial tumours (prostate cancer, urinary bladder cancer, and kidney cancer). A size-based separation method for viable CTC - enrichment from unclothed peripheral blood has been introduced (MetaCell, Ostrava, Czech Republic). The enriched CTCs fraction was cultured directly on the separation membrane, or transferred from the membrane and cultured on any plastic surface or a microscopic slide.
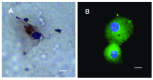
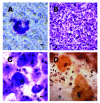
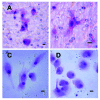
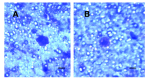
Results: We report a successful application of a CTCs isolation procedure in patients with urothelial cancers. The CTCs captured on the membrane are enriched with a remarkable proliferation potential. This has enabled us to set up in vitro cell cultures from the viable CTCs unaffected by any fixation buffers, antibodies or lysing solutions. Next, the CTCs were cultured in vitro for a minimum of 10 to 14 days to enable further downstream analysis (e.g., immunohistochemistry).
Conclusion: We demonstrated an efficient CTCs capture platform, based on a cell size separation principle. Furthermore, we report an ability to culture the enriched cells - a critical requirement for post-isolation cellular analysis.
Figures



References
-
- Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–5. - PubMed
-
- Fidler IJ. Metastasis: Guantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. J. Natl. Cancer Inst. 1970;45:773–82. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
