Production of transgenic pigs over-expressing the antiviral gene Mx1
- PMID: 25408889
- PMCID: PMC4230515
- DOI: 10.1186/2045-9769-3-11
Production of transgenic pigs over-expressing the antiviral gene Mx1
Abstract
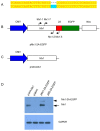
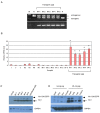
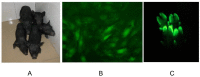
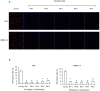
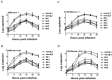
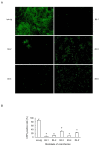
The myxovirus resistance gene (Mx1) has a broad spectrum of antiviral activities. It is therefore an interesting candidate gene to improve disease resistance in farm animals. In this study, we report the use of somatic cell nuclear transfer (SCNT) to produce transgenic pigs over-expressing the Mx1 gene. These transgenic pigs express approximately 15-25 times more Mx1 mRNA than non-transgenic pigs, and the protein level of Mx1 was also markedly enhanced. We challenged fibroblast cells isolated from the ear skin of transgenic and control pigs with influenza A virus and classical swine fever virus (CFSV). Indirect immunofluorescence assay (IFA) revealed a profound decrease of influenza A proliferation in Mx1 transgenic cells. Growth kinetics showed an approximately 10-fold reduction of viral copies in the transgenic cells compared to non-transgenic controls. Additionally, we found that the Mx1 transgenic cells were more resistant to CSFV infection in comparison to non-transgenic cells. These results demonstrate that the Mx1 transgene can protect against viral infection in cells of transgenic pigs and indicate that the Mx1 transgene can be harnessed to develop disease-resistant pigs.
Keywords: Antiviral breeding; Innate resistance; Somatic cell nuclear transfer.
Figures
Similar articles
-
Transgenic pigs carrying cDNA copies encoding the murine Mx1 protein which confers resistance to influenza virus infection.Gene. 1992 Nov 16;121(2):263-70. doi: 10.1016/0378-1119(92)90130-h. Gene. 1992. PMID: 1446823
-
Resistance to influenza virus infection of Mx transgenic mice expressing Mx protein under the control of two constitutive promoters.J Virol. 1992 Mar;66(3):1709-16. doi: 10.1128/JVI.66.3.1709-1716.1992. J Virol. 1992. PMID: 1371172 Free PMC article.
-
Porcine Mx1 Protein Inhibits Classical Swine Fever Virus Replication by Targeting Nonstructural Protein NS5B.J Virol. 2018 Mar 14;92(7):e02147-17. doi: 10.1128/JVI.02147-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343573 Free PMC article.
-
Classical swine fever virus replicated poorly in cells from MxA transgenic pigs.BMC Vet Res. 2016 Aug 17;12(1):169. doi: 10.1186/s12917-016-0794-5. BMC Vet Res. 2016. PMID: 27535023 Free PMC article.
-
Mx proteins: GTPases involved in the interferon-induced antiviral state.Ciba Found Symp. 1993;176:233-43; discussion 243-7. doi: 10.1002/9780470514450.ch15. Ciba Found Symp. 1993. PMID: 7507812 Review.
Cited by
-
Current and prospective control strategies of influenza A virus in swine.Porcine Health Manag. 2021 Feb 28;7(1):23. doi: 10.1186/s40813-021-00196-0. Porcine Health Manag. 2021. PMID: 33648602 Free PMC article. Review.
-
Generation of PCBP1-deficient pigs using CRISPR/Cas9-mediated gene editing.iScience. 2022 Oct 3;25(10):105268. doi: 10.1016/j.isci.2022.105268. eCollection 2022 Oct 21. iScience. 2022. PMID: 36274935 Free PMC article.
-
Extensive germline genome engineering in pigs.Nat Biomed Eng. 2021 Feb;5(2):134-143. doi: 10.1038/s41551-020-00613-9. Epub 2020 Sep 21. Nat Biomed Eng. 2021. PMID: 32958897
-
Synthetic RNAs Mimicking Structural Domains in the Foot-and-Mouth Disease Virus Genome Elicit a Broad Innate Immune Response in Porcine Cells Triggered by RIG-I and TLR Activation.Viruses. 2015 Jul 17;7(7):3954-73. doi: 10.3390/v7072807. Viruses. 2015. PMID: 26193305 Free PMC article.
-
A Wide-Ranging Antiviral Response in Wild Boar Cells Is Triggered by Non-coding Synthetic RNAs From the Foot-and-Mouth Disease Virus Genome.Front Vet Sci. 2020 Aug 4;7:495. doi: 10.3389/fvets.2020.00495. eCollection 2020. Front Vet Sci. 2020. PMID: 32851049 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

