Arvicanthis ansorgei, a Novel Model for the Study of Sleep and Waking in Diurnal Rodents
- PMID: 25409107
- PMCID: PMC4434565
- DOI: 10.5665/sleep.4754
Arvicanthis ansorgei, a Novel Model for the Study of Sleep and Waking in Diurnal Rodents
Abstract
Study objectives: Sleep neurobiology studies use nocturnal species, mainly rats and mice. However, because their daily sleep/wake organization is inverted as compared to humans, a diurnal model for sleep studies is needed. To fill this gap, we phenotyped sleep and waking in Arvicanthis ansorgei, a diurnal rodent widely used for the study of circadian rhythms.
Design: Video-electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG) recordings.
Setting: Rodent sleep laboratory.
Participants: Fourteen male Arvicanthis ansorgei, aged 3 mo.
Interventions: 12 h light (L):12 h dark (D) baseline condition, 24-h constant darkness, 6-h sleep deprivation.
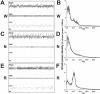
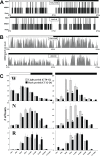
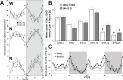
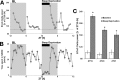
Measurements and results: Wake and rapid eye movement (REM) sleep showed similar electrophysiological characteristics as nocturnal rodents. On average, animals spent 12.9 h ± 0.4 awake per 24-h cycle, of which 6.88 h ± 0.3 was during the light period. NREM sleep accounted for 9.63 h ± 0.4, which of 5.13 h ± 0.2 during dark period, and REM sleep for 89.9 min ± 6.7, which of 52.8 min ± 4.4 during dark period. The time-course of sleep and waking across the 12 h light:12 h dark was overall inverted to that observed in rats or mice, though with larger amounts of crepuscular activity at light and dark transitions. A dominant crepuscular regulation of sleep and waking persisted under constant darkness, showing the lack of a strong circadian drive in the absence of clock reinforcement by external cues, such as a running wheel. Conservation of the homeostatic regulation was confirmed with the observation of higher delta power following sustained waking periods and a 6-h sleep deprivation, with subsequent decrease during recovery sleep.
Conclusions: Arvicanthis ansorgei is a valid diurnal rodent model for studying the regulatory mechanisms of sleep and so represents a valuable tool for further understanding the nocturnality/diurnality switch.
Keywords: Arvicanthis ansorgei; circadian rhythm; crepuscular; direct effects of light; diurnality; nocturnality; rodent; sleep deprivation; sleep homeostasis; sleep regulation.
© 2015 Associated Professional Sleep Societies, LLC.
Figures


References
-
- Dijk DJ, Daan S. Sleep EEG spectral analysis in a diurnal rodent: Eutamias sibiricus. J Comp Physiol A. 1989;165:205–15. - PubMed
-
- Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol. 1977;233:R213–21. - PubMed
-
- Kas MJ, Edgar DM. Crepuscular rhythms of EEG sleep-wake in a hystricomorph rodent, Octodon degus. J Biol Rhythms. 1998;13:9–17. - PubMed
-
- Challet E, Pitrosky B, Sicard B, Malan A, Pevet P. Circadian organization in a diurnal rodent, Arvicanthis ansorgei Thomas 1910: chronotypes, responses to constant lighting conditions, and photoperiodic changes. J Biol Rhythms. 2002;1:52–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

