Involvement of IKAP in peripheral target innervation and in specific JNK and NGF signaling in developing PNS neurons
- PMID: 25409162
- PMCID: PMC4237409
- DOI: 10.1371/journal.pone.0113428
Involvement of IKAP in peripheral target innervation and in specific JNK and NGF signaling in developing PNS neurons
Abstract
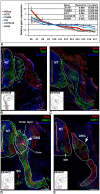
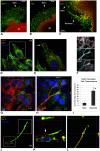
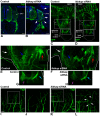
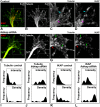
A splicing mutation in the ikbkap gene causes Familial Dysautonomia (FD), affecting the IKAP protein expression levels and proper development and function of the peripheral nervous system (PNS). Here we attempted to elucidate the role of IKAP in PNS development in the chick embryo and found that IKAP is required for proper axonal outgrowth, branching, and peripheral target innervation. Moreover, we demonstrate that IKAP colocalizes with activated JNK (pJNK), dynein, and β-tubulin at the axon terminals of dorsal root ganglia (DRG) neurons, and may be involved in transport of specific target derived signals required for transcription of JNK and NGF responsive genes in the nucleus. These results suggest the novel role of IKAP in neuronal transport and specific signaling mediated transcription, and provide, for the first time, the basis for a molecular mechanism behind the FD phenotype.
Conflict of interest statement
Figures
Similar articles
-
Familial Dysautonomia (FD) Human Embryonic Stem Cell Derived PNS Neurons Reveal that Synaptic Vesicular and Neuronal Transport Genes Are Directly or Indirectly Affected by IKBKAP Downregulation.PLoS One. 2015 Oct 5;10(10):e0138807. doi: 10.1371/journal.pone.0138807. eCollection 2015. PLoS One. 2015. PMID: 26437462 Free PMC article.
-
Phosphatidylserine Ameliorates Neurodegenerative Symptoms and Enhances Axonal Transport in a Mouse Model of Familial Dysautonomia.PLoS Genet. 2016 Dec 20;12(12):e1006486. doi: 10.1371/journal.pgen.1006486. eCollection 2016 Dec. PLoS Genet. 2016. PMID: 27997532 Free PMC article.
-
IKAP/Elp1 is required in vivo for neurogenesis and neuronal survival, but not for neural crest migration.PLoS One. 2012;7(2):e32050. doi: 10.1371/journal.pone.0032050. Epub 2012 Feb 23. PLoS One. 2012. PMID: 22384137 Free PMC article.
-
Familial dysautonomia.Curr Opin Genet Dev. 2002 Jun;12(3):307-11. doi: 10.1016/s0959-437x(02)00303-9. Curr Opin Genet Dev. 2002. PMID: 12076674 Review.
-
Familial dysautonomia.Muscle Nerve. 2004 Mar;29(3):352-63. doi: 10.1002/mus.10499. Muscle Nerve. 2004. PMID: 14981733 Review.
Cited by
-
ELP1, the Gene Mutated in Familial Dysautonomia, Is Required for Normal Enteric Nervous System Development and Maintenance and for Gut Epithelium Homeostasis.J Neurosci. 2024 Sep 11;44(37):e2253232024. doi: 10.1523/JNEUROSCI.2253-23.2024. J Neurosci. 2024. PMID: 39138000 Free PMC article.
-
Carotid baroreflex activation therapy for resistant hypertension.Nat Rev Cardiol. 2015 Aug;12(8):451-63. doi: 10.1038/nrcardio.2015.96. Epub 2015 Jul 7. Nat Rev Cardiol. 2015. PMID: 26149485 Review.
-
Elp1 is required for development of visceral sensory peripheral and central circuitry.Dis Model Mech. 2022 May 1;15(5):dmm049274. doi: 10.1242/dmm.049274. Epub 2022 Jun 1. Dis Model Mech. 2022. PMID: 35481599 Free PMC article.
-
Familial Dysautonomia (FD) Human Embryonic Stem Cell Derived PNS Neurons Reveal that Synaptic Vesicular and Neuronal Transport Genes Are Directly or Indirectly Affected by IKBKAP Downregulation.PLoS One. 2015 Oct 5;10(10):e0138807. doi: 10.1371/journal.pone.0138807. eCollection 2015. PLoS One. 2015. PMID: 26437462 Free PMC article.
-
ATP-citrate lyase promotes axonal transport across species.Nat Commun. 2021 Oct 7;12(1):5878. doi: 10.1038/s41467-021-25786-y. Nat Commun. 2021. PMID: 34620845 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

