Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp
- PMID: 25409186
- PMCID: PMC4237368
- DOI: 10.1371/journal.pone.0112518
Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp
Abstract
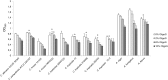
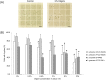
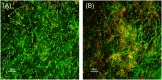
The oligosaccharide OligoG, an alginate derived from seaweed, has been shown to have anti-bacterial and anti-biofilm properties and potentiates the activity of selected antibiotics against multi-drug resistant bacteria. The ability of OligoG to perturb fungal growth and potentiate conventional antifungal agents was evaluated using a range of pathogenic fungal strains. Candida (n = 11) and Aspergillus (n = 3) spp. were tested using germ tube assays, LIVE/DEAD staining, scanning electron microscopy (SEM), atomic force microscopy (AFM) and high-throughput minimum inhibition concentration assays (MICs). In general, the strains tested showed a significant dose-dependent reduction in cell growth at ≥6% OligoG as measured by optical density (OD600; P<0.05). OligoG (>0.5%) also showed a significant inhibitory effect on hyphal growth in germ tube assays, although strain-dependent variations in efficacy were observed (P<0.05). SEM and AFM both showed that OligoG (≥2%) markedly disrupted fungal biofilm formation, both alone, and in combination with fluconazole. Cell surface roughness was also significantly increased by the combination treatment (P<0.001). High-throughput robotic MIC screening demonstrated the potentiating effects of OligoG (2, 6, 10%) with nystatin, amphotericin B, fluconazole, miconazole, voriconazole or terbinafine with the test strains. Potentiating effects were observed for the Aspergillus strains with all six antifungal agents, with an up to 16-fold (nystatin) reduction in MIC. Similarly, all the Candida spp. showed potentiation with nystatin (up to 16-fold) and fluconazole (up to 8-fold). These findings demonstrate the antifungal properties of OligoG and suggest a potential role in the management of fungal infections and possible reduction of antifungal toxicity.
Conflict of interest statement
Figures


Similar articles
-
Alginate oligosaccharides enhance the antifungal activity of nystatin against candidal biofilms.Front Cell Infect Microbiol. 2023 Jan 31;13:1122340. doi: 10.3389/fcimb.2023.1122340. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36798083 Free PMC article.
-
Alginate oligosaccharides modify hyphal infiltration of Candida albicans in an in vitro model of invasive human candidosis.J Appl Microbiol. 2017 Sep;123(3):625-636. doi: 10.1111/jam.13516. Epub 2017 Aug 1. J Appl Microbiol. 2017. PMID: 28635170
-
A nanoscale characterization of the interaction of a novel alginate oligomer with the cell surface and motility of Pseudomonas aeruginosa.Am J Respir Cell Mol Biol. 2014 Mar;50(3):483-92. doi: 10.1165/rcmb.2013-0287OC. Am J Respir Cell Mol Biol. 2014. PMID: 24074505
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Antifungal drug resistance in pathogenic fungi.Med Mycol. 1998;36 Suppl 1:119-28. Med Mycol. 1998. PMID: 9988500 Review.
Cited by
-
Does the Freeze-Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug?Int J Mol Sci. 2022 Jun 17;23(12):6775. doi: 10.3390/ijms23126775. Int J Mol Sci. 2022. PMID: 35743216 Free PMC article.
-
Mucoadhesive Alginate/Pectin Films Crosslinked by Calcium Carbonate as Carriers of a Model Antifungal Drug-Posaconazole.Pharmaceutics. 2023 Oct 3;15(10):2415. doi: 10.3390/pharmaceutics15102415. Pharmaceutics. 2023. PMID: 37896175 Free PMC article.
-
Alginate Oligosaccharide-Induced Modification of the lasI-lasR and rhlI-rhlR Quorum-Sensing Systems in Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02318-17. doi: 10.1128/AAC.02318-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463534 Free PMC article.
-
The antimicrobial effects of the alginate oligomer OligoG CF-5/20 are independent of direct bacterial cell membrane disruption.Sci Rep. 2017 Mar 31;7:44731. doi: 10.1038/srep44731. Sci Rep. 2017. PMID: 28361894 Free PMC article.
-
Exploiting Mannuronan C-5 Epimerases in Commercial Alginate Production.Mar Drugs. 2020 Nov 18;18(11):565. doi: 10.3390/md18110565. Mar Drugs. 2020. PMID: 33218095 Free PMC article.
References
-
- Lass-Florl C (2009) The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 52: 197–205. - PubMed
-
- Ramage G, Martinez JP, Lopez-Ribot JL (2006) Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6: 979–986. - PubMed
-
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, et al. (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36: 288–305. - PubMed
-
- Alcazar-Fuoli L, Mellado E (2014) Current status of antifungal resistance and its impact on clinical practice. Br J Haematol 166: 471–484. - PubMed
-
- Guinea J (2014) Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20: 5–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

