Long-term safety and efficacy of factor IX gene therapy in hemophilia B
- PMID: 25409372
- PMCID: PMC4278802
- DOI: 10.1056/NEJMoa1407309
Long-term safety and efficacy of factor IX gene therapy in hemophilia B
Abstract
Background: In patients with severe hemophilia B, gene therapy that is mediated by a novel self-complementary adeno-associated virus serotype 8 (AAV8) vector has been shown to raise factor IX levels for periods of up to 16 months. We wanted to determine the durability of transgene expression, the vector dose-response relationship, and the level of persistent or late toxicity.
Methods: We evaluated the stability of transgene expression and long-term safety in 10 patients with severe hemophilia B: 6 patients who had been enrolled in an initial phase 1 dose-escalation trial, with 2 patients each receiving a low, intermediate, or high dose, and 4 additional patients who received the high dose (2×10(12) vector genomes per kilogram of body weight). The patients subsequently underwent extensive clinical and laboratory monitoring.
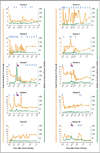
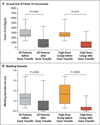
Results: A single intravenous infusion of vector in all 10 patients with severe hemophilia B resulted in a dose-dependent increase in circulating factor IX to a level that was 1 to 6% of the normal value over a median period of 3.2 years, with observation ongoing. In the high-dose group, a consistent increase in the factor IX level to a mean (±SD) of 5.1±1.7% was observed in all 6 patients, which resulted in a reduction of more than 90% in both bleeding episodes and the use of prophylactic factor IX concentrate. A transient increase in the mean alanine aminotransferase level to 86 IU per liter (range, 36 to 202) occurred between week 7 and week 10 in 4 of the 6 patients in the high-dose group but resolved over a median of 5 days (range, 2 to 35) after prednisolone treatment.
Conclusions: In 10 patients with severe hemophilia B, the infusion of a single dose of AAV8 vector resulted in long-term therapeutic factor IX expression associated with clinical improvement. With a follow-up period of up to 3 years, no late toxic effects from the therapy were reported. (Funded by the National Heart, Lung, and Blood Institute and others; ClinicalTrials.gov number, NCT00979238.).
Figures
Comment in
-
Moving forward toward a cure for hemophilia B.Mol Ther. 2015 May;23(5):809-811. doi: 10.1038/mt.2015.56. Mol Ther. 2015. PMID: 25943496 Free PMC article. No abstract available.
-
Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice.Gene Ther. 2016 Jun;23(6):510-9. doi: 10.1038/gt.2016.23. Epub 2016 Apr 7. Gene Ther. 2016. PMID: 26960037 Free PMC article.
References
-
- Nathwani AC, Tuddenham EG. Epidemiology of coagulation disorders. Baillieres Clin Haematol. 1992;5:383–439. - PubMed
-
- Ponder KP, Srivastava A. Walk a mile in the moccasins of people with haemophilia. Haemophilia. 2008;14:618–620. - PubMed
-
- Pipe SW. The hope and reality of long-acting hemophilia products. Am J Hematol. 2012;87(Suppl 1):S33–S39. - PubMed
-
- Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
