Amyotrophic lateral sclerosis-linked mutant VAPB inclusions do not interfere with protein degradation pathways or intracellular transport in a cultured cell model
- PMID: 25409455
- PMCID: PMC4237408
- DOI: 10.1371/journal.pone.0113416
Amyotrophic lateral sclerosis-linked mutant VAPB inclusions do not interfere with protein degradation pathways or intracellular transport in a cultured cell model
Abstract
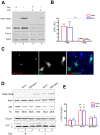
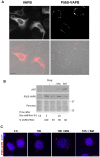
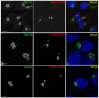
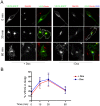
VAPB is a ubiquitously expressed, ER-resident adaptor protein involved in interorganellar lipid exchange, membrane contact site formation, and membrane trafficking. Its mutant form, P56S-VAPB, which has been linked to a dominantly inherited form of Amyotrophic Lateral Sclerosis (ALS8), generates intracellular inclusions consisting in restructured ER domains whose role in ALS pathogenesis has not been elucidated. P56S-VAPB is less stable than the wild-type protein and, at variance with most pathological aggregates, its inclusions are cleared by the proteasome. Based on studies with cultured cells overexpressing the mutant protein, it has been suggested that VAPB inclusions may exert a pathogenic effect either by sequestering the wild-type protein and other interactors (loss-of-function by a dominant negative effect) or by a more general proteotoxic action (gain-of-function). To investigate P56S-VAPB degradation and the effect of the inclusions on proteostasis and on ER-to-plasma membrane protein transport in a more physiological setting, we used stable HeLa and NSC34 Tet-Off cell lines inducibly expressing moderate levels of P56S-VAPB. Under basal conditions, P56S-VAPB degradation was mediated exclusively by the proteasome in both cell lines, however, it could be targeted also by starvation-stimulated autophagy. To assess possible proteasome impairment, the HeLa cell line was transiently transfected with the ERAD (ER Associated Degradation) substrate CD3δ, while autophagic flow was investigated in cells either starved or treated with an autophagy-stimulating drug. Secretory pathway functionality was evaluated by analyzing the transport of transfected Vesicular Stomatitis Virus Glycoprotein (VSVG). P56S-VAPB expression had no effect either on the degradation of CD3δ or on the levels of autophagic markers, or on the rate of transport of VSVG to the cell surface. We conclude that P56S-VAPB inclusions expressed at moderate levels do not interfere with protein degradation pathways or protein transport, suggesting that the dominant inheritance of the mutant gene may be due mainly to haploinsufficiency.
Conflict of interest statement
Figures


Similar articles
-
Restructured endoplasmic reticulum generated by mutant amyotrophic lateral sclerosis-linked VAPB is cleared by the proteasome.J Cell Sci. 2012 Aug 1;125(Pt 15):3601-11. doi: 10.1242/jcs.102137. Epub 2012 May 18. J Cell Sci. 2012. PMID: 22611258
-
Amyotrophic lateral sclerosis (ALS)-associated VAPB-P56S inclusions represent an ER quality control compartment.Acta Neuropathol Commun. 2013 Jun 12;1:24. doi: 10.1186/2051-5960-1-24. Acta Neuropathol Commun. 2013. PMID: 24252306 Free PMC article.
-
A VAPB mutant linked to amyotrophic lateral sclerosis generates a novel form of organized smooth endoplasmic reticulum.FASEB J. 2010 May;24(5):1419-30. doi: 10.1096/fj.09-147850. Epub 2009 Dec 14. FASEB J. 2010. PMID: 20008544
-
The Link between VAPB Loss of Function and Amyotrophic Lateral Sclerosis.Cells. 2021 Jul 23;10(8):1865. doi: 10.3390/cells10081865. Cells. 2021. PMID: 34440634 Free PMC article. Review.
-
ER stress and unfolded protein response in amyotrophic lateral sclerosis.Mol Neurobiol. 2009 Apr;39(2):81-9. doi: 10.1007/s12035-009-8054-3. Epub 2009 Jan 30. Mol Neurobiol. 2009. PMID: 19184563 Review.
Cited by
-
SOD1 activity threshold and TOR signalling modulate VAP(P58S) aggregation via reactive oxygen species-induced proteasomal degradation in a Drosophila model of amyotrophic lateral sclerosis.Dis Model Mech. 2019 Feb 7;12(2):dmm033803. doi: 10.1242/dmm.033803. Dis Model Mech. 2019. PMID: 30635270 Free PMC article.
-
A role for mitochondria-ER crosstalk in amyotrophic lateral sclerosis 8 pathogenesis.Life Sci Alliance. 2025 Jan 27;8(4):e202402907. doi: 10.26508/lsa.202402907. Print 2025 Apr. Life Sci Alliance. 2025. PMID: 39870504 Free PMC article.
-
Golgi Fragmentation in ALS Motor Neurons. New Mechanisms Targeting Microtubules, Tethers, and Transport Vesicles.Front Neurosci. 2015 Dec 8;9:448. doi: 10.3389/fnins.2015.00448. eCollection 2015. Front Neurosci. 2015. PMID: 26696811 Free PMC article. Review.
-
Autophagy and Neurodegeneration: Insights from a Cultured Cell Model of ALS.Cells. 2015 Aug 6;4(3):354-86. doi: 10.3390/cells4030354. Cells. 2015. PMID: 26287246 Free PMC article. Review.
-
Overexpression of mTOR in Leukocytes from ALS8 Patients.Curr Neuropharmacol. 2023;21(3):482-490. doi: 10.2174/1570159X21666230201151016. Curr Neuropharmacol. 2023. PMID: 36722478 Free PMC article.
References
-
- Lev S, Ben Halevy D, Peretti D, Dahan N (2008) The VAP protein family: from cellular functions to motor neuron disease. Trends Cell Biol 18: 282–290. - PubMed
-
- Kawano M, Kumagai K, Nishijima M, Hanada K (2006) Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem 281: 30279–30288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

