Immunohistochemistry of colorectal cancer biomarker phosphorylation requires controlled tissue fixation
- PMID: 25409462
- PMCID: PMC4237459
- DOI: 10.1371/journal.pone.0113608
Immunohistochemistry of colorectal cancer biomarker phosphorylation requires controlled tissue fixation
Abstract
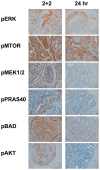
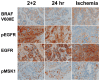
Phosphorylated signaling molecules are biomarkers of cancer pathophysiology and resistance to therapy, but because phosphoprotein analytes are often labile, poorly controlled clinical laboratory practices could prevent translation of research findings in this area from the bench to the bedside. We therefore compared multiple biomarker and phosphoprotein immunohistochemistry (IHC) results in 23 clinical colorectal carcinoma samples after either a novel, rapid tissue fixation protocol or a standard tissue fixation protocol employed by clinical laboratories, and we also investigated the effect of a defined post-operative "cold" ischemia period on these IHC results. We found that a one-hour cold ischemia interval, allowed by ASCO/CAP guidelines for certain cancer biomarker assays, is highly deleterious to certain phosphoprotein analytes, specifically the phosphorylated epidermal growth factor receptor (pEGFR), but shorter ischemic intervals (less than 17 minutes) facilitate preservation of phosphoproteins. Second, we found that a rapid 4-hour, two temperature, formalin fixation yielded superior staining in several cases with select markers (pEGFR, pBAD, pAKT) compared to a standard overnight room temperature fixation protocol, despite taking less time. These findings indicate that the future research and clinical utilities of phosphoprotein IHC for assessing colorectal carcinoma pathophysiology absolutely depend upon attention to preanalytical factors and rigorously controlled tissue fixation protocols.
Conflict of interest statement
Figures


References
-
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, et al. (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131: 18–43. - PubMed
-
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, et al. (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134: e48–72. - PubMed
-
- Hewitt S, Robinowitz M, Bogen S, Gown A, Kalra K, et al. (2011) Quality Assurance for Design Control and Implementation of Immunohistochemistry Assays; Approved Guideline – Second Edition, CLSI Document I/LA28-A2. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute.
-
- Yildiz-Aktas IZ, Dabbs DJ, Bhargava R (2012) The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol 25: 1098–1105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

