Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo
- PMID: 25409543
- PMCID: PMC4238015
- DOI: 10.1038/srep07134
Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo
Erratum in
-
CORRIGENDUM: Different Inhibitory Effect and Mechanism of Hydroxyapatite Nanoparticles on Normal Cells and Cancer Cells In Vitro and In Vivo.Sci Rep. 2015 Jan 27;5:7943. doi: 10.1038/srep07943. Sci Rep. 2015. PMID: 25623075 Free PMC article. No abstract available.
Abstract
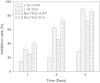
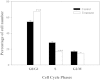
Hydroxyapatite (HAP), similar to inorganic phase in bones, shows good biocompatibility and bioactivity as bone defect repairing material. Recently, nanoscaled HAP shows the special properties differing from bulk HAP in physics, chemistry and biology. This paper demonstrates that HAP nanoparticle (nHAP) possesses the ability for inhibiting cancer cell growth in vitro and in vivo. In vitro, after treatment with nHAP for 3 days, proliferation of human cancer cells are inhibited by more than 65% and by less than 30% for human normal cells. In vivo, injection of nHAP in transplanted tumor results in significant reduction (about 50%) of tumor size. The anticancer effect of nHAP is mainly attributed to high amount by endocytosis in cancer cells and inhibition on protein synthesis in cells. The abundant nHAP internalized in cancer cells around endoplasmic reticulum may inhibit the protein synthesis by decreasing the binding of mRNA to ribosome due to its high adsorption capacity for ribosome and arrest cell cycle in G0/G1 phase. nHAP shows no ROS-involved cytotoxicity and low cytotoxicity to normal cells. These results strongly suggest that nHAP can inhibit cancer cell proliferation and have a potential application in cancer treatment.
Figures





References
-
- LeGeros R. Z. Calcium phosphates in oral biology and medicine. (Karger: Basel, Switzerland, 1991). - PubMed
-
- Aoki H. Science and medical applications of hydroxyapatite. (Japanese Association of Apatite Science, Tokyo, 1991).
-
- Vallet-Regí M. & González-Calbet J. M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 32, 1–31 (2004).
-
- Meyers M. A., Mishra A. & Benson D. J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 51, 427–556 (2006).
-
- Li H., Khor K. A., Chow V. & Cheang P. Nanostructural characteristics, mechanical properties, and osteoblast response of spark plasma sintered hydroxyapatite. J. Biomed. Mater. Res. Part A 82, 296–303 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

