Fas Activated Serine-Threonine Kinase Domains 2 (FASTKD2) mediates apoptosis of breast and prostate cancer cells through its novel FAST2 domain
- PMID: 25409762
- PMCID: PMC4256816
- DOI: 10.1186/1471-2407-14-852
Fas Activated Serine-Threonine Kinase Domains 2 (FASTKD2) mediates apoptosis of breast and prostate cancer cells through its novel FAST2 domain
Abstract
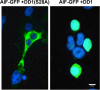
Background: Expression of NRIF3 (Nuclear Receptor Interacting Factor-3) rapidly and selectively leads to apoptosis of breast cancer cells. This occurs through binding of NRIF3 or its 30 amino acid Death Domain-1 (DD1) region to the transcriptional repressor, DIF-1 (DD1 Interacting Factor-1). DIF-1 acts in a wide variety of breast cancer cells but not other cell types to repress the pro-apoptotic gene, FASTKD2. Expression of NRIF3 or DD1 inactivates the DIF-1 repressor leading to rapid derepression of FASTKD2, which initiates apoptosis within 5-8 h of expression. Although FASTKD2 is an inner mitochondrial membrane protein, it does not require mitochondrial localization to initiate apoptosis.
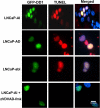
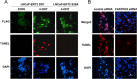
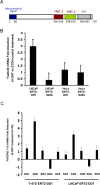
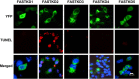
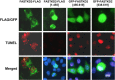
Methods: Androgen dependent LNCaP cells as well as two androgen independent LNCaP cell lines (LNCaP-AI and LNCaP-abl) were studied and LNCaP-AI cells were engineered to conditionally express DD1 or the inactive DD1-S28A with 4-hydroxytamoxifen. Apoptosis was assessed by TUNEL assay. FASTKD2 is related to 4 other proteins encoded in the human genome (FASTKD1, 3, 4, 5). All contain a poorly conserved putative bipartite kinase domain designated as FAST1_FAST2. We examined whether expression of any of the other FASTKD isoforms leads to apoptosis and sought to identify the region of FASTKD2 necessary to initiate the apoptotic pathway.
Results: Of the FASTKD1-5 isoforms only expression of FASTKD2 leads to apoptosis. Although, the NRIF3/DD1/DIF-1 pathway does not mediate apoptosis of a wide variety of non-breast cancer cell lines, because of certain similarities and gene signatures between breast and prostate cancer we explored whether the NRIF3/DD1/DIF-1/FASTKD2 pathway mediates apoptosis of prostate cancer cells. We found that the pathway leads to apoptosis in LNCaP cells, including the two androgen-independent LNCaP cell lines that are generally resistant to apoptosis. Lastly, we identified that FASTKD2-mediated apoptosis is initiated by the 81 amino acid FAST2 region.
Conclusions: The NRIF3/DIF-1/FASTKD2 pathway acts as a "death switch" in breast and prostate cancer cells. Deciphering how this pathway is regulated and how FASTKD2 initiates the apoptotic response will allow for the development of therapeutic agents for the treatment of androgen-independent prostate cancer or Tamoxifen-unresponsive Estrogen Receptor negative tumors as well as metastatic breast or prostate cancer.
Figures
References
-
- Das S, Nwachukwu JC, Li D, Vulin AI, Martinez-Caballero S, Kinnally KW, Samuels HH. The nuclear receptor interacting factor-3 transcriptional coregulator mediates rapid apoptosis in breast cancer cells through direct and bystander-mediated events. Cancer Res. 2007;67:1775–1782. doi: 10.1158/0008-5472.CAN-06-4034. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/852/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

