A comparative encyclopedia of DNA elements in the mouse genome
- PMID: 25409824
- PMCID: PMC4266106
- DOI: 10.1038/nature13992
A comparative encyclopedia of DNA elements in the mouse genome
Abstract
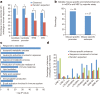
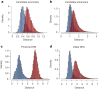
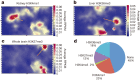
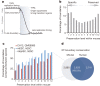
The laboratory mouse shares the majority of its protein-coding genes with humans, making it the premier model organism in biomedical research, yet the two mammals differ in significant ways. To gain greater insights into both shared and species-specific transcriptional and cellular regulatory programs in the mouse, the Mouse ENCODE Consortium has mapped transcription, DNase I hypersensitivity, transcription factor binding, chromatin modifications and replication domains throughout the mouse genome in diverse cell and tissue types. By comparing with the human genome, we not only confirm substantial conservation in the newly annotated potential functional sequences, but also find a large degree of divergence of sequences involved in transcriptional regulation, chromatin state and higher order chromatin organization. Our results illuminate the wide range of evolutionary forces acting on genes and their regulatory regions, and provide a general resource for research into mammalian biology and mechanisms of human diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Chinwalla AT, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 CA165863/CA/NCI NIH HHS/United States
- U01 HL099656/HL/NHLBI NIH HHS/United States
- R01 HG007175/HG/NHGRI NIH HHS/United States
- 095908/WT_/Wellcome Trust/United Kingdom
- P01 GM085354/GM/NIGMS NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R37 DK044746/DK/NIDDK NIH HHS/United States
- R01 HG007348/HG/NHGRI NIH HHS/United States
- U01 HG004695/HG/NHGRI NIH HHS/United States
- K99HL119617/HL/NHLBI NIH HHS/United States
- U54 HG007004/HG/NHGRI NIH HHS/United States
- F32 HL110473/HL/NHLBI NIH HHS/United States
- R01 HG004037/HG/NHGRI NIH HHS/United States
- R01 DK096266/DK/NIDDK NIH HHS/United States
- RC2 HG005573/HG/NHGRI NIH HHS/United States
- K99 HG007356/HG/NHGRI NIH HHS/United States
- R01HD043997-09/HD/NICHD NIH HHS/United States
- R56 DK065806/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- MC_UU_12009/2/MRC_/Medical Research Council/United Kingdom
- F32HL110473/HL/NHLBI NIH HHS/United States
- 1U54HG007004/HG/NHGRI NIH HHS/United States
- R01 HD043997/HD/NICHD NIH HHS/United States
- 3RC2HG005602/HG/NHGRI NIH HHS/United States
- F31CA165863/CA/NCI NIH HHS/United States
- K99 HL119617/HL/NHLBI NIH HHS/United States
- R01HG003991/HG/NHGRI NIH HHS/United States
- P01 HL064190/HL/NHLBI NIH HHS/United States
- GM085354/GM/NIGMS NIH HHS/United States
- R01 DK065806/DK/NIDDK NIH HHS/United States
- RC2HG005573/HG/NHGRI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- R01 ES024992/ES/NIEHS NIH HHS/United States
- T32 GM081739/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 HL099993/HL/NHLBI NIH HHS/United States
- R01DK065806/DK/NIDDK NIH HHS/United States
- P01 HL110860/HL/NHLBI NIH HHS/United States
- U54 HG006997/HG/NHGRI NIH HHS/United States
- R01 GM083337/GM/NIGMS NIH HHS/United States
- R01 HG003991/HG/NHGRI NIH HHS/United States
- GM083337/GM/NIGMS NIH HHS/United States
- RC2 HG005602/HG/NHGRI NIH HHS/United States
- R01 EY021482/EY/NEI NIH HHS/United States
- R01 HG007354/HG/NHGRI NIH HHS/United States
- U54 HG006998/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

