Insights into glycogen metabolism in Lactobacillus acidophilus: impact on carbohydrate metabolism, stress tolerance and gut retention
- PMID: 25410006
- PMCID: PMC4243779
- DOI: 10.1186/s12934-014-0094-3
Insights into glycogen metabolism in Lactobacillus acidophilus: impact on carbohydrate metabolism, stress tolerance and gut retention
Abstract
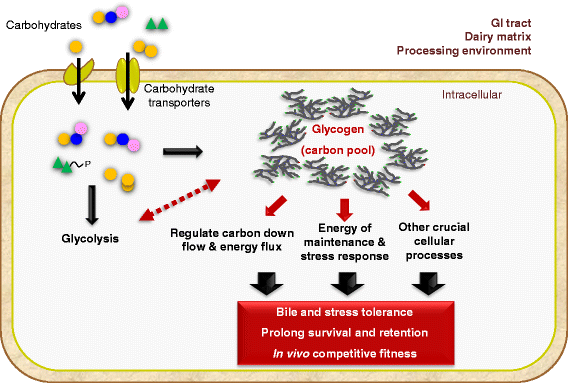
In prokaryotic species equipped with glycogen metabolism machinery, the co-regulation of glycogen biosynthesis and degradation has been associated with the synthesis of energy storage compounds and various crucial physiological functions, including global cellular processes such as carbon and nitrogen metabolism, energy sensing and production, stress response and cell-cell communication. In addition, the glycogen metabolic pathway was proposed to serve as a carbon capacitor that regulates downstream carbon fluxes, and in some microorganisms the ability to synthesize intracellular glycogen has been implicated in host persistence. Among lactobacilli, complete glycogen metabolic pathway genes are present only in select species predominantly associated with mammalian hosts or natural environments. This observation highlights the potential involvement of glycogen biosynthesis in probiotic activities and persistence of intestinal lactobacilli in the human gastrointestinal tract. In this review, we summarize recent findings on (i) the presence and potential ecological distribution of glycogen metabolic pathways among lactobacilli, (ii) influence of carbon substrates and growth phases on glycogen metabolic gene expression and glycogen accumulation in L. acidophilus, and (iii) the involvement of glycogen metabolism on growth, sugar utilization and bile tolerance. Our present in vivo studies established the significance of glycogen biosynthesis on the competitive retention of L. acidophilus in the mouse intestinal tract, demonstrating for the first time that the ability to synthesize intracellular glycogen contributes to gut fitness and retention among probiotic microorganisms.
Figures


Similar articles
-
A functional glycogen biosynthesis pathway in Lactobacillus acidophilus: expression and analysis of the glg operon.Mol Microbiol. 2013 Sep;89(6):1187-200. doi: 10.1111/mmi.12338. Epub 2013 Aug 16. Mol Microbiol. 2013. PMID: 23879596 Free PMC article.
-
Lactobacillus acidophilus Metabolizes Dietary Plant Glucosides and Externalizes Their Bioactive Phytochemicals.mBio. 2017 Nov 21;8(6):e01421-17. doi: 10.1128/mBio.01421-17. mBio. 2017. PMID: 29162708 Free PMC article.
-
An Extracellular Cell-Attached Pullulanase Confers Branched α-Glucan Utilization in Human Gut Lactobacillus acidophilus.Appl Environ Microbiol. 2017 May 31;83(12):e00402-17. doi: 10.1128/AEM.00402-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28411221 Free PMC article.
-
Intracellular glycogen accumulation by human gut commensals as a niche adaptation trait.Gut Microbes. 2023 Jan-Dec;15(1):2235067. doi: 10.1080/19490976.2023.2235067. Gut Microbes. 2023. PMID: 37526383 Free PMC article. Review.
-
Glycogen and its metabolism: some new developments and old themes.Biochem J. 2012 Feb 1;441(3):763-87. doi: 10.1042/BJ20111416. Biochem J. 2012. PMID: 22248338 Free PMC article. Review.
Cited by
-
Feruloyl Esterase (LaFae) from Lactobacillus acidophilus: Structural Insights and Functional Characterization for Application in Ferulic Acid Production.Int J Mol Sci. 2023 Jul 6;24(13):11170. doi: 10.3390/ijms241311170. Int J Mol Sci. 2023. PMID: 37446348 Free PMC article.
-
Physiological and Molecular Responses to Main Environmental Stressors of Microalgae and Bacteria in Polar Marine Environments.Microorganisms. 2020 Dec 9;8(12):1957. doi: 10.3390/microorganisms8121957. Microorganisms. 2020. PMID: 33317109 Free PMC article. Review.
-
Food, Health, and Environmental Impact of Lactic Acid Bacteria: The Superbacteria for Posterity.Probiotics Antimicrob Proteins. 2025 Apr 28. doi: 10.1007/s12602-025-10546-x. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40289239 Review.
-
Two Species of Long-Day Breeding Hamsters Exhibit Distinct Gut Microbial Responses to Photoperiodic Variations.Animals (Basel). 2025 Jun 3;15(11):1648. doi: 10.3390/ani15111648. Animals (Basel). 2025. PMID: 40509114 Free PMC article.
-
Early life microbiota transplantation from highly feed-efficient broiler improved weight gain by reshaping the gut microbiota in laying chicken.Front Microbiol. 2022 Nov 18;13:1022783. doi: 10.3389/fmicb.2022.1022783. eCollection 2022. Front Microbiol. 2022. PMID: 36466637 Free PMC article.
References
-
- Preiss J. Encyclopedia of microbiology (Schaechter M ed. 3. Oxford: Elsevier; 2009. Glycogen biosynthesis; pp. 145–158.
-
- Alonso-Casajus N, Dauvillee D, Viale AM, Munoz FJ, Baroja-Fernandez E, Moran-Zorzano MT, Eydallin G, Ball S, Pozueta-Romero J. Glycogen phosphorylase, the product of the glgP gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J Bacteriol. 2006;188:5266–5272. doi: 10.1128/JB.01566-05. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

