Vesicles derived via AP-3-dependent recycling contribute to asynchronous release and influence information transfer
- PMID: 25410111
- PMCID: PMC4239664
- DOI: 10.1038/ncomms6530
Vesicles derived via AP-3-dependent recycling contribute to asynchronous release and influence information transfer
Abstract
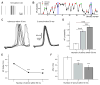
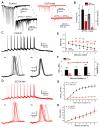
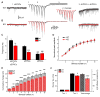
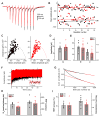
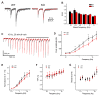
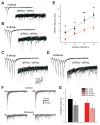
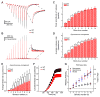
Action potentials trigger synchronous and asynchronous neurotransmitter release. Temporal properties of both types of release could be altered in an activity-dependent manner. While the effects of activity-dependent changes in synchronous release on postsynaptic signal integration have been studied, the contribution of asynchronous release to information transfer during natural stimulus patterns is unknown. Here we find that during trains of stimulations, asynchronous release contributes to the precision of action potential firing. Our data show that this form of release is selectively diminished in AP-3b2 KO animals, which lack functional neuronal AP-3, an adaptor protein regulating vesicle formation from endosomes generated during bulk endocytosis. We find that in the absence of neuronal AP-3, asynchronous release is attenuated and the activity-dependent increase in the precision of action potential timing is compromised. Lack of asynchronous release decreases the capacity of synaptic information transfer and renders synaptic communication less reliable in response to natural stimulus patterns.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

