HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer
- PMID: 25410163
- PMCID: PMC4717359
- DOI: 10.1136/gutjnl-2014-307918
HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer
Abstract
Background: Worldwide, gastric cancer (GC) is the fourth most common malignancy and the most common cancer in East Asia. Development of targeted therapies for this disease has focused on a few known oncogenes but has had limited effects.
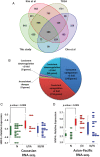
Objective: To determine oncogenic mechanisms and novel therapeutic targets specific for GC by identifying commonly dysregulated genes from the tumours of both Asian-Pacific and Caucasian patients.
Methods: We generated transcriptomic profiles of 22 Caucasian GC tumours and their matched non-cancerous samples and performed an integrative analysis across different GC gene expression datasets. We examined the inhibition of commonly overexpressed oncogenes and their constituent signalling pathways by RNAi and/or pharmacological inhibition.
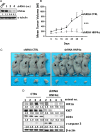
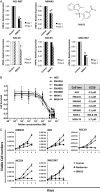
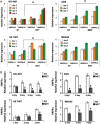
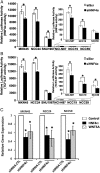
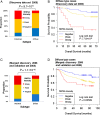
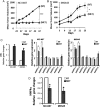
Results: Hepatocyte nuclear factor-4α (HNF4α) upregulation was a key signalling event in gastric tumours from both Caucasian and Asian patients, and HNF4α antagonism was antineoplastic. Perturbation experiments in GC tumour cell lines and xenograft models further demonstrated that HNF4α is downregulated by AMPKα signalling and the AMPK agonist metformin; blockade of HNF4α activity resulted in cyclin downregulation, cell cycle arrest and tumour growth inhibition. HNF4α also regulated WNT signalling through its target gene WNT5A, a potential prognostic marker of diffuse type gastric tumours.
Conclusions: Our results indicate that HNF4α is a targetable oncoprotein in GC, is regulated by AMPK signalling through AMPKα and resides upstream of WNT signalling. HNF4α may regulate 'metabolic switch' characteristic of a general malignant phenotype and its target WNT5A has potential prognostic values. The AMPKα-HNF4α-WNT5A signalling cascade represents a potentially targetable pathway for drug development.
Keywords: DRUG DEVELOPMENT; GASTRIC CANCER; GENE EXPRESSION; MOLECULAR BIOLOGY; ONCOGENES.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. - PubMed
-
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. - PubMed
-
- McDonald M, Hertz RP, Pitman Lowenthal SW. Pfizer facts, the burden of cancer in Asia. USA: Pfizer, 2008.
-
- Ferlay JEA. GLOBOCAN 2008, cancer incidence and mortality worldwide. CancerBase 2010;2010.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
