Global displacement of canine parvovirus by a host-adapted variant: structural comparison between pandemic viruses with distinct host ranges
- PMID: 25410876
- PMCID: PMC4300749
- DOI: 10.1128/JVI.02611-14
Global displacement of canine parvovirus by a host-adapted variant: structural comparison between pandemic viruses with distinct host ranges
Abstract
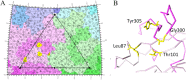
Canine parvovirus type 2 (CPV-2) emerged in 1978 and spread worldwide within 2 years. Subsequently, CPV-2 was completely replaced by the variant CPV-2a, which is characterized by four specific capsid (VP2) mutations. The X-ray crystal structure of the CPV-2a capsid shows that each mutation confers small local changes. The loss of a hydrogen bond and introduction of a glycine residue likely introduce flexibility to sites that control interactions with the host receptor, antibodies, and sialic acids.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

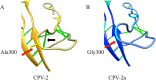
References
-
- Barker IK, Parrish CR. 2001. Parvovirus infections, p 131–146 InWilliams ES, Barker IK (ed), Infectious diseases of wild mammals, 3rd ed Iowa State University Press, Ames, IA.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

