Protease-activated receptor 2 activation inhibits N-type Ca2+ currents in rat peripheral sympathetic neurons
- PMID: 25410909
- PMCID: PMC4255100
- DOI: 10.14348/molcells.2014.0167
Protease-activated receptor 2 activation inhibits N-type Ca2+ currents in rat peripheral sympathetic neurons
Abstract
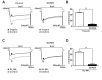
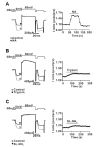
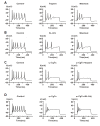
The protease-activated receptor (PAR)-2 is highly expressed in endothelial cells and vascular smooth muscle cells. It plays a crucial role in regulating blood pressure via the modulation of peripheral vascular tone. Although several mechanisms have been suggested to explain PAR-2-induced hypotension, the precise mechanism remains to be elucidated. To investigate this possibility, we investigated the effects of PAR-2 activation on N-type Ca(2+) currents (I(Ca-N)) in isolated neurons of the celiac ganglion (CG), which is involved in the sympathetic regulation of mesenteric artery vascular tone. PAR-2 agonists irreversibly diminished voltage-gated Ca(2+) currents (I(Ca)), measured using the patch-clamp method, in rat CG neurons, whereas thrombin had little effect on I(Ca). This PAR-2-induced inhibition was almost completely prevented by ω-CgTx, a potent N-type Ca(2+) channel blocker, suggesting the involvement of N-type Ca(2+) channels in PAR-2-induced inhibition. In addition, PAR-2 agonists inhibited I(Ca-N) in a voltage-independent manner in rat CG neurons. Moreover, PAR-2 agonists reduced action potential (AP) firing frequency as measured using the current-clamp method in rat CG neurons. This inhibition of AP firing induced by PAR-2 agonists was almost completely prevented by ω-CgTx, indicating that PAR-2 activation may regulate the membrane excitability of peripheral sympathetic neurons through modulation of N-type Ca(2+) channels. In conclusion, the present findings demonstrate that the activation of PAR-2 suppresses peripheral sympathetic outflow by modulating N-type Ca(2+) channel activity, which appears to be involved in PAR-2-induced hypotension, in peripheral sympathetic nerve terminals.
Keywords: N-type Ca2+ channel; celiac ganglion; hypotension; peripheral sympathetic output; protease-activated receptor 2.
Figures


Similar articles
-
Suppression of peripheral sympathetic activity underlies protease-activated receptor 2-mediated hypotension.Korean J Physiol Pharmacol. 2014 Dec;18(6):489-95. doi: 10.4196/kjpp.2014.18.6.489. Epub 2014 Dec 30. Korean J Physiol Pharmacol. 2014. PMID: 25598663 Free PMC article.
-
Modulation of N-type calcium currents by presynaptic imidazoline receptor activation in rat superior cervical ganglion neurons.Exp Physiol. 2010 Oct;95(10):982-93. doi: 10.1113/expphysiol.2010.053355. Epub 2010 Aug 9. Exp Physiol. 2010. PMID: 20696781
-
Modulation of N-type Ca²⁺ currents by moxonidine via imidazoline I₁ receptor activation in rat superior cervical ganglion neurons.Biochem Biophys Res Commun. 2011 Jun 17;409(4):645-50. doi: 10.1016/j.bbrc.2011.05.058. Epub 2011 May 17. Biochem Biophys Res Commun. 2011. PMID: 21620797
-
Agmatine suppresses peripheral sympathetic tone by inhibiting N-type Ca(2+) channel activity via imidazoline I2 receptor activation.Biochem Biophys Res Commun. 2016 Aug 26;477(3):406-12. doi: 10.1016/j.bbrc.2016.06.086. Epub 2016 Jun 16. Biochem Biophys Res Commun. 2016. PMID: 27320860
-
Increase of L-type Ca2+ current by protease-activated receptor 2 activation contributes to augmentation of spontaneous uterine contractility in pregnant rats.Biochem Biophys Res Commun. 2012 Feb 3;418(1):167-72. doi: 10.1016/j.bbrc.2011.12.154. Epub 2012 Jan 8. Biochem Biophys Res Commun. 2012. PMID: 22244874
Cited by
-
Diverse impact of acute and long-term extracellular proteolytic activity on plasticity of neuronal excitability.Front Cell Neurosci. 2015 Aug 10;9:313. doi: 10.3389/fncel.2015.00313. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26321914 Free PMC article. Review.
References
-
- al-Ani B., Saifeddine M., Hollenberg M.D. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. - PubMed
-
- Carrier G.O., Ikeda S.R. TTX-sensitive Na+ channels and Ca2+ channels of the L- and N-type underlie the inward current in acutely dispersed coeliac-mesenteric ganglia neurons of adult rats. Pflugers Arch. 1992;421:7–16. - PubMed
-
- Cheung W.M., Andrade-Gordon P., Derian C.K., Damiano B.P. Receptor-activating peptides distinguish thrombin receptor (PAR-1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can. J. Physiol. Pharmacol. 1998;76:16–25. - PubMed
-
- Chien E.K., Sweet L., Phillippe M., Marietti S., Kim T.T., Wolff D.A., Thomas L., Bieber E. Protease-activated receptor isoform expression in pregnant and nonpregnant rat myometrial tissue. J. Soc. Gynecol. Investig. 2003;10:460–468. - PubMed
-
- Chung S., Ahn D.S., Kim Y.H., Kim Y.S., Joeng J.H., Nam T.S. Modulation of N-type calcium currents by presynaptic imidazoline receptor activation in rat superior cervical ganglion neurons. Exp. Physiol. 2010;95:982–993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

