Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors
- PMID: 25411085
- PMCID: PMC4406150
- DOI: 10.1002/cncr.29155
Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors
Abstract
Background: The current phase 1, open-label, dose escalation study was conducted to establish the safety, tolerability, pharmacokinetic profile, and preliminary antitumor activity of the novel mitochondrial inhibitor ME-344 in patients with refractory solid tumors.
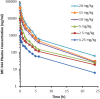
Methods: Patients with refractory solid tumors were treated in a 3 + 3 dose escalation design. ME-344 was administered via intravenous infusion on days 1, 8, and 15 of the first 28-day cycle and weekly thereafter. Pharmacokinetics was assessed on days 1 and 15 of the first cycle.
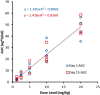
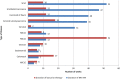
Results: A total of 30 patients (median age, 65 years; 67% of whom were female) received ME-344. There were 5 dose-limiting toxicities reported. Four patients developed grade 3 neuropathy (2 patients each at doses of 15 mg/kg and 20 mg/kg) and 1 patient treated at a dose of 10 mg/kg developed a grade 3 acute myocardial infarction (toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events [version 4.03]). The maximum tolerated dose (MTD) was defined as 10 mg/kg weekly. The most common adverse events were nausea, dizziness, and fatigue. At the MTD of 10 mg/kg, the maximal plasma concentration (Cmax) was 25.8 µg/mL and the area under the concentration curve from time zero to infinity was 25.9 hour*µg/mL. One patient with small cell lung cancer achieved a partial response for ≥ 52 weeks. Four patients had prolonged stable disease (1 patient each with urothelial carcinoma [47 weeks], carcinoid tumor [≥ 40 weeks], cervical leiomyosarcoma [39 weeks], and cervical cancer [≥ 31 weeks]).
Conclusions: The once-weekly administration of ME-344 was generally well tolerated in the current study, a first-in-human study; dose-limiting neuropathy was noted, but not at the MTD. Exposures at the 10-mg/kg dose level suggest a sufficient therapeutic index. The preliminary clinical activity as a monotherapy supports the further clinical development of ME-344 in combination with chemotherapy.
Keywords: ME-344; first-in-human; maximum tolerated dose (MTD); mitochondrial inhibitor; phase 1; refractory solid tumors.
© 2014 American Cancer Society.
Figures

References
-
- Alvero AB, Sumi N, Craveiro V. ME-344 delays tumor kinetics in an ovarian cancer in vivo recurrence model [abstract] Cancer Res. 2013;73:Page. et al.. Abstract LB-286.
-
- Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guidelines (version 1.1) Eur J Cancer. 2009;45:228–247. et al. - PubMed
-
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 2010. . Version 4.03. Washington, DC: US Department of Health and Human Services;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

