Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro
- PMID: 25411113
- PMCID: PMC4694114
- DOI: 10.1111/joa.12257
Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro
Abstract
Research in mammalian cell biology often relies on developing in vitro models to enable the growth of cells in the laboratory to investigate a specific biological mechanism or process under different test conditions. The quality of such models and how they represent the behavior of cells in real tissues plays a critical role in the value of the data produced and how it is used. It is particularly important to recognize how the structure of a cell influences its function and how co-culture models can be used to more closely represent the structure of real tissue. In recent years, technologies have been developed to enhance the way in which researchers can grow cells and more readily create tissue-like structures. Here we identify the limitations of culturing mammalian cells by conventional methods on two-dimensional (2D) substrates and review the popular approaches currently available that enable the development of three-dimensional (3D) tissue models in vitro. There are now many ways in which the growth environment for cultured cells can be altered to encourage 3D cell growth. Approaches to 3D culture can be broadly categorized into scaffold-free or scaffold-based culture systems, with scaffolds made from either natural or synthetic materials. There is no one particular solution that currently satisfies all requirements and researchers must select the appropriate method in line with their needs. Using such technology in conjunction with other modern resources in cell biology (e.g. human stem cells) will provide new opportunities to create robust human tissue mimetics for use in basic research and drug discovery. Application of such models will contribute to advancing basic research, increasing the predictive accuracy of compounds, and reducing animal usage in biomedical science.
Keywords: 3D cell culture; in vitro; technology; tissue structure.
© 2014 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures
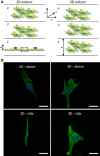
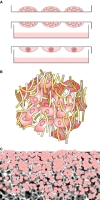


References
-
- Abe K, Niwa H, Iwase K, et al. (1996) Endoderm‐specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp Cell Res 229, 27–34. - PubMed
-
- Antonchuk J (2013) Formation of embryoid bodies from human pluripotent stem cells using AggreWell plates. Methods Mol Biol, 946, 523–533. - PubMed
-
- Awad HA, Wickham MQ, Leddy HA, et al. (2004) Chondrogenic differentiation of adipose‐derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25, 3211–3222. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

