Quantitative analysis of the cellular composition in seminiferous tubules in normal and genetically modified infertile mice
- PMID: 25411188
- PMCID: PMC4305514
- DOI: 10.1369/0022155414562045
Quantitative analysis of the cellular composition in seminiferous tubules in normal and genetically modified infertile mice
Abstract
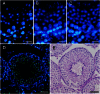
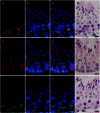
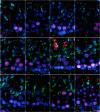
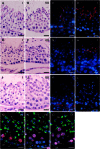
The aim of this study was to establish a quantitative standard for the cellular composition in seminiferous tubules at each stage of spermatogenesis in the mouse testis, and thereby evaluate abnormalities in the infertile mouse testis. We applied a combination of lectin histochemistry for acrosomes and immunohistochemistry for various specific cell markers, both of which were visualized with fluorescence, on paraffin sections of the testis. We first examined seminiferous tubules from normal mice and counted the number of each cell type at each stage of spermatogenesis. We then examined seminiferous tubules from genetically modified mice deficient (-/-) for one of the cell adhesion molecules, nectin-2 or nectin-3, and compared the number of each cell type at each stage of spermatogenesis with the corresponding value in normal mice. In both nectin-2-/- and nectin-3-/- mice, which are infertile despite the apparently normal morphology of the seminiferous epithelia, we measured a progressive loss in the later-step spermatids, with significantly lower numbers of step 11-16 spermatids in nectin-3-/- mice and step 15-16 spermatids in nectin-2-/- mice as compared with that in normal control mice. The present study demonstrated that a quantitative analysis of cellular compositions at different stages in seminiferous tubules was useful for evaluating abnormalities in spermatogenesis.
Keywords: cell adhesion molecule; fluorescence; immunohistochemistry; lectin; spermatogenesis; stage.
© The Author(s) 2014.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Abercrombie M. (1946). Estimation of nuclear population from microtome sections. Anat Rec 94:239-247. - PubMed
-
- Ahmed EA, de Rooij DG. (2009). Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol 558:263-277. - PubMed
-
- Arya M, Vanha-Perttula T. (1986). Comparison of lectin-staining pattern in testis and epididymis of gerbil, guinea pig, mouse, and nutria. Am J Anat 175:449-469. - PubMed
-
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. (2004). Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36:647-652. - PubMed
-
- Chiarini-Garcia H, Russell LD. (2001). High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod 65:1170–1178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

