Altered lymph node composition in diphtheria toxin receptor-based mouse models to ablate dendritic cells
- PMID: 25411201
- PMCID: PMC4272857
- DOI: 10.4049/jimmunol.1401999
Altered lymph node composition in diphtheria toxin receptor-based mouse models to ablate dendritic cells
Abstract
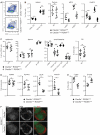
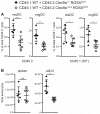
Dendritic cells (DCs) are key regulators of innate and adaptive immunity. Our understanding of immune function has benefited greatly from mouse models allowing for selective ablation of DCs. Many such models rely on transgenic diphtheria toxin receptor (DTR) expression driven by DC-restricted promoters. This renders DCs sensitive to DT but is otherwise thought to have no effect on immune physiology. In this study, we report that, unexpectedly, mice in which DTR is expressed on conventional DCs display marked lymph node (LN) hypocellularity and reduced frequency of DCs in the same organs but not in spleen or nonlymphoid tissues. Intriguingly, in mixed bone marrow chimeras the phenotype conferred by DTR-expressing DCs is dominant over control bone marrow-derived cells, leading to small LNs and an overall paucity of DCs independently of the genetic ability to express DTR. The finding of alterations in LN composition and size independently of DT challenge suggests that caution must be exercised when interpreting results of experiments obtained with mouse models to inducibly deplete DCs. It further indicates that DTR, a member of the epidermal growth factor family, is biologically active in mice. Its use in cell ablation experiments needs to be considered in light of this activity.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



References
-
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. - PubMed
-
- Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. - PubMed
-
- Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, Rogers NC, Moncaut N, Carvajal JJ, Reis e Sousa C. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell. 2013;154:843–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

