Human NAT10 is an ATP-dependent RNA acetyltransferase responsible for N4-acetylcytidine formation in 18 S ribosomal RNA (rRNA)
- PMID: 25411247
- PMCID: PMC4276842
- DOI: 10.1074/jbc.C114.602698
Human NAT10 is an ATP-dependent RNA acetyltransferase responsible for N4-acetylcytidine formation in 18 S ribosomal RNA (rRNA)
Abstract
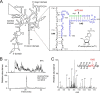
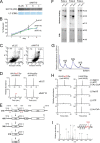
Human N-acetyltransferase 10 (NAT10) is known to be a lysine acetyltransferase that targets microtubules and histones and plays an important role in cell division. NAT10 is highly expressed in malignant tumors, and is also a promising target for therapies against laminopathies and premature aging. Here we report that NAT10 is an ATP-dependent RNA acetyltransferase responsible for formation of N(4)-acetylcytidine (ac(4)C) at position 1842 in the terminal helix of mammalian 18 S rRNA. RNAi-mediated knockdown of NAT10 resulted in growth retardation of human cells, and this was accompanied by high-level accumulation of the 30 S precursor of 18 S rRNA, suggesting that ac(4)C1842 formation catalyzed by NAT10 is involved in rRNA processing and ribosome biogenesis.
Keywords: Acetyl Coenzyme A (Acetyl-CoA); Acetyltransferase; RNA Modification; Ribosomal RNA Processing (rRNA Processing); Ribosome Assembly.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Mullineux S. T., Lafontaine D. L. (2012) Mapping the cleavage sites on mammalian pre-rRNAs: where do we stand? Biochimie 94, 1521–1532 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

