Role of renal sensory nerves in physiological and pathophysiological conditions
- PMID: 25411364
- PMCID: PMC4297860
- DOI: 10.1152/ajpregu.00351.2014
Role of renal sensory nerves in physiological and pathophysiological conditions
Abstract
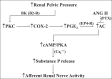
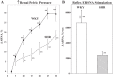
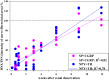
Whether activation of afferent renal nerves contributes to the regulation of arterial pressure and sodium balance has been long overlooked. In normotensive rats, activating renal mechanosensory nerves decrease efferent renal sympathetic nerve activity (ERSNA) and increase urinary sodium excretion, an inhibitory renorenal reflex. There is an interaction between efferent and afferent renal nerves, whereby increases in ERSNA increase afferent renal nerve activity (ARNA), leading to decreases in ERSNA by activation of the renorenal reflexes to maintain low ERSNA to minimize sodium retention. High-sodium diet enhances the responsiveness of the renal sensory nerves, while low dietary sodium reduces the responsiveness of the renal sensory nerves, thus producing physiologically appropriate responses to maintain sodium balance. Increased renal ANG II reduces the responsiveness of the renal sensory nerves in physiological and pathophysiological conditions, including hypertension, congestive heart failure, and ischemia-induced acute renal failure. Impairment of inhibitory renorenal reflexes in these pathological states would contribute to the hypertension and sodium retention. When the inhibitory renorenal reflexes are suppressed, excitatory reflexes may prevail. Renal denervation reduces arterial pressure in experimental hypertension and in treatment-resistant hypertensive patients. The fall in arterial pressure is associated with a fall in muscle sympathetic nerve activity, suggesting that increased ARNA contributes to increased arterial pressure in these patients. Although removal of both renal sympathetic and afferent renal sensory nerves most likely contributes to the arterial pressure reduction initially, additional mechanisms may be involved in long-term arterial pressure reduction since sympathetic and sensory nerves reinnervate renal tissue in a similar time-dependent fashion following renal denervation.
Keywords: angiotensin; hypertension; kidney; prostaglandin E2; renal denervation; renal mechanosensory nerves; substance P.
Copyright © 2015 the American Physiological Society.
Figures








References
-
- Almgård LE, Ljungqvist A, Ungerstedt U. The reaction of the intrarenal sympathetic nervous system to renal transplantion. Scand J Urol Nephrol 5: 65–70, 1971. - PubMed
-
- Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol 70: 735–749, 1992. - PubMed
-
- Barajas L, Powers K. Innervation of the renal proximal convoluted tubule of the rat. Am J Anat 186: 378–388, 1989. - PubMed
-
- Barajas L, Powers K. Monoaminergic innervation of the rat kidney: a quantitative study. Am J Physiol Renal Fluid Electrolyte Physiol 259: F503–F511, 1990. - PubMed
-
- Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. Am J Physiol Renal Fluid Electrolyte Physiol 247: F50–F60, 1984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

