Multi-haem cytochromes in Shewanella oneidensis MR-1: structures, functions and opportunities
- PMID: 25411412
- PMCID: PMC4277109
- DOI: 10.1098/rsif.2014.1117
Multi-haem cytochromes in Shewanella oneidensis MR-1: structures, functions and opportunities
Abstract
Multi-haem cytochromes are employed by a range of microorganisms to transport electrons over distances of up to tens of nanometres. Perhaps the most spectacular utilization of these proteins is in the reduction of extracellular solid substrates, including electrodes and insoluble mineral oxides of Fe(III) and Mn(III/IV), by species of Shewanella and Geobacter. However, multi-haem cytochromes are found in numerous and phylogenetically diverse prokaryotes where they participate in electron transfer and redox catalysis that contributes to biogeochemical cycling of N, S and Fe on the global scale. These properties of multi-haem cytochromes have attracted much interest and contributed to advances in bioenergy applications and bioremediation of contaminated soils. Looking forward, there are opportunities to engage multi-haem cytochromes for biological photovoltaic cells, microbial electrosynthesis and developing bespoke molecular devices. As a consequence, it is timely to review our present understanding of these proteins and we do this here with a focus on the multitude of functionally diverse multi-haem cytochromes in Shewanella oneidensis MR-1. We draw on findings from experimental and computational approaches which ideally complement each other in the study of these systems: computational methods can interpret experimentally determined properties in terms of molecular structure to cast light on the relation between structure and function. We show how this synergy has contributed to our understanding of multi-haem cytochromes and can be expected to continue to do so for greater insight into natural processes and their informed exploitation in biotechnologies.
Keywords: Marcus theory; cytochrome; electron transfer; haem; redox potential; respiration.
© 2014 The Author(s) Published by the Royal Society. All rights reserved.
Figures
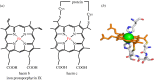
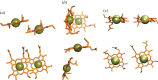
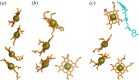

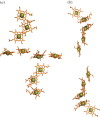
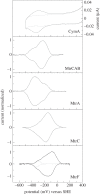

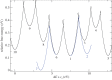
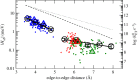
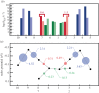
 for each pair (circles, area proportional to the coupling). The colour code of the circles corresponds to the three haem pair motifs as in figure 11. (Adapted from [115].)
for each pair (circles, area proportional to the coupling). The colour code of the circles corresponds to the three haem pair motifs as in figure 11. (Adapted from [115].)

Similar articles
-
Interaction studies between periplasmic cytochromes provide insights into extracellular electron transfer pathways of Geobacter sulfurreducens.Biochem J. 2017 Feb 20;474(5):797-808. doi: 10.1042/BCJ20161022. Biochem J. 2017. PMID: 28093471
-
Dissimilatory Fe(III) and Mn(IV) reduction.Adv Microb Physiol. 2004;49:219-86. doi: 10.1016/S0065-2911(04)49005-5. Adv Microb Physiol. 2004. PMID: 15518832 Review.
-
Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes.Mol Microbiol. 2007 Jul;65(1):12-20. doi: 10.1111/j.1365-2958.2007.05783.x. Mol Microbiol. 2007. PMID: 17581116 Free PMC article. Review.
-
Nanosecond heme-to-heme electron transfer rates in a multiheme cytochrome nanowire reported by a spectrally unique His/Met-ligated heme.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2107939118. doi: 10.1073/pnas.2107939118. Proc Natl Acad Sci U S A. 2021. PMID: 34556577 Free PMC article.
-
Nature's conductors: what can microbial multi-heme cytochromes teach us about electron transport and biological energy conversion?Curr Opin Chem Biol. 2018 Dec;47:7-17. doi: 10.1016/j.cbpa.2018.06.007. Epub 2018 Jul 14. Curr Opin Chem Biol. 2018. PMID: 30015234 Review.
Cited by
-
Periplasmic Protein Mobility for Extracellular Electron Transport in Shewanella oneidensis.Microorganisms. 2025 May 16;13(5):1144. doi: 10.3390/microorganisms13051144. Microorganisms. 2025. PMID: 40431315 Free PMC article.
-
Secreted Cysteine-Rich Repeat Proteins "SCREPs": A Novel Multi-Domain Architecture.Front Pharmacol. 2018 Nov 20;9:1333. doi: 10.3389/fphar.2018.01333. eCollection 2018. Front Pharmacol. 2018. PMID: 30524283 Free PMC article.
-
A Microbial Electrochemical Technology to Detect and Degrade Organophosphate Pesticides.ACS Cent Sci. 2021 Oct 27;7(10):1718-1727. doi: 10.1021/acscentsci.1c00931. Epub 2021 Sep 21. ACS Cent Sci. 2021. PMID: 34729415 Free PMC article.
-
Kinetics of trifurcated electron flow in the decaheme bacterial proteins MtrC and MtrF.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3425-3430. doi: 10.1073/pnas.1818003116. Epub 2019 Feb 12. Proc Natl Acad Sci U S A. 2019. PMID: 30755526 Free PMC article.
-
Flavin Binding to the Deca-heme Cytochrome MtrC: Insights from Computational Molecular Simulation.Biophys J. 2015 Dec 15;109(12):2614-2624. doi: 10.1016/j.bpj.2015.10.038. Biophys J. 2015. PMID: 26682818 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- BB/C007808/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G009228/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K009885/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G007519/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

