Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding
- PMID: 25411488
- PMCID: PMC6608438
- DOI: 10.1523/JNEUROSCI.2664-14.2014
Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding
Abstract
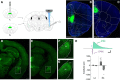
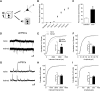
Conditioned fear requires neural activity in the basolateral amygdala (BLA) and medial prefrontal cortex (mPFC), structures that are densely interconnected at the synaptic level. Previous work has suggested that anatomical subdivisions of mPFC make distinct contributions to fear expression and inhibition, and that the functional output of this processing is relayed to the BLA complex. However, it remains unknown whether synaptic plasticity in mPFC-BLA networks contributes to fear memory encoding. Here we use optogenetics and ex vivo electrophysiology to reveal the impact of fear conditioning on BLA excitatory and feedforward inhibitory circuits formed by projections from infralimbic (IL) and prelimbic (PL) cortices. In naive mice, these pathways recruit equivalent excitation and feedforward inhibition in BLA principal neurons. However, fear learning leads to a selective decrease in inhibition:excitation balance in PL circuits that is attributable to a postsynaptic increase in AMPA receptor function. These data suggest a pathway-specific mechanism for fear memory encoding by adjustment of mPFC-BLA transmission. Upon reengagement of PL by conditioned cues, these modifications may serve to amplify emotional responses.
Keywords: amygdala; channelrhodopsin; fear; memory; prefrontal; synaptic plasticity.
Copyright © 2014 the authors 0270-6474/14/3415601-09$15.00/0.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
