Netrin-G/NGL complexes encode functional synaptic diversification
- PMID: 25411505
- PMCID: PMC6608433
- DOI: 10.1523/JNEUROSCI.1141-14.2014
Netrin-G/NGL complexes encode functional synaptic diversification
Abstract
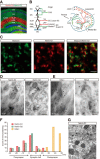
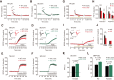
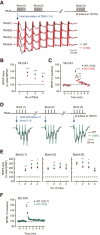
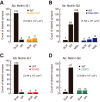
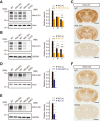
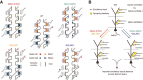
Synaptic cell adhesion molecules are increasingly gaining attention for conferring specific properties to individual synapses. Netrin-G1 and netrin-G2 are trans-synaptic adhesion molecules that distribute on distinct axons, and their presence restricts the expression of their cognate receptors, NGL1 and NGL2, respectively, to specific subdendritic segments of target neurons. However, the neural circuits and functional roles of netrin-G isoform complexes remain unclear. Here, we use netrin-G-KO and NGL-KO mice to reveal that netrin-G1/NGL1 and netrin-G2/NGL2 interactions specify excitatory synapses in independent hippocampal pathways. In the hippocampal CA1 area, netrin-G1/NGL1 and netrin-G2/NGL2 were expressed in the temporoammonic and Schaffer collateral pathways, respectively. The lack of presynaptic netrin-Gs led to the dispersion of NGLs from postsynaptic membranes. In accord, netrin-G mutant synapses displayed opposing phenotypes in long-term and short-term plasticity through discrete biochemical pathways. The plasticity phenotypes in netrin-G-KOs were phenocopied in NGL-KOs, with a corresponding loss of netrin-Gs from presynaptic membranes. Our findings show that netrin-G/NGL interactions differentially control synaptic plasticity in distinct circuits via retrograde signaling mechanisms and explain how synaptic inputs are diversified to control neuronal activity.
Keywords: excitatory synapse; mice; netrin-G1; netrin-G2; pathway specificity; trans-synaptic adhesion molecule.
Copyright © 2014 the authors 0270-6474/14/3415779-14$15.00/0.
Figures



References
-
- Aoki-Suzuki M, Yamada K, Meerabux J, Iwayama-Shigeno Y, Ohba H, Iwamoto K, Takao H, Toyota T, Suto Y, Nakatani N, Dean B, Nishimura S, Seki K, Kato T, Itohara S, Nishikawa T, Yoshikawa T. A family-based association study and gene expression analyses of netrin-G1 and -G2 genes in schizophrenia. Biol Psychiatry. 2005;57:382–393. doi: 10.1016/j.biopsych.2004.11.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
