Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome
- PMID: 25411640
- PMCID: PMC4236430
- DOI: 10.1186/s40425-014-0040-2
Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome
Abstract
Background: Investigate the tumor diameter and density changes in advanced melanoma patients treated with ipilimumab plus bevacizumab, compare response rates based on different response criteria, and study association between these measures and survival.
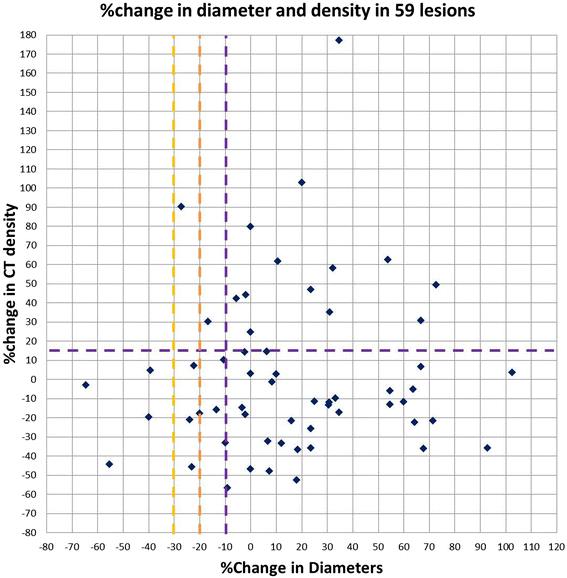
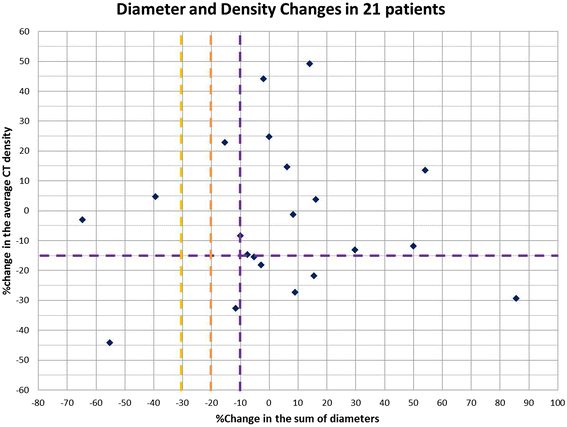
Methods: Twenty-one advanced melanoma patients with 59 measurable lesions treated in a phase 1 trial of ipilimumab plus bevacizumab were retrospectively studied. Tumor diameter and density were measured on baseline and first follow-up CT. Responses were assigned using RECIST, MASS and Choi criteria. Diameter and density measures and responses by these criteria were studied for the association with survival.
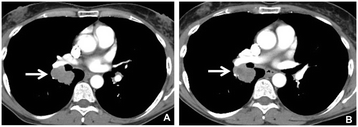
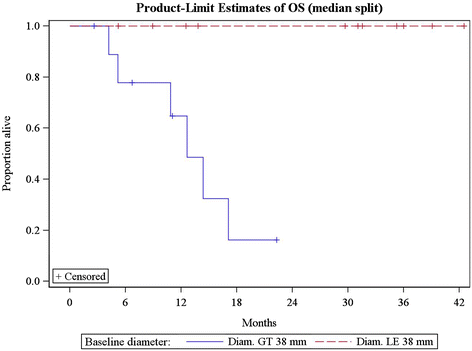
Results: Twenty-three (39%) lesions and 7 (33%) patients met the Choi density criteria for response (≥15% density decrease) at the first follow-up. The response rates were 14% (3/21, 95% CI: 3-36%) by RECIST and MASS, and 52% (11/21, 95% CI: 30-74%) by Choi criteria, when both size and density criteria were used. Larger baseline tumor diameter was significantly associated with shorter progression-free survival (PFS) and overall survival (OS) (log-rank p = 0.001 and 0.003; respectively). Diameter or density changes, or responses by RECIST, MASS or Choi criteria at the first follow-up, were not associated with PFS or OS.
Conclusion: Tumor density decrease meeting Choi criteria was noted in one-third of advanced melanoma patients at the first follow-up scan during ipilimumab plus bevacizumab therapy. While larger baseline tumor diameter was strongly associated with shorter survival, changes of diameter or density, or responses by three criteria did not predict survival. The role of density changes in evaluating response during ipilimumab and bevacizumab therapy for advanced melanoma remains to be further established.
Keywords: Anti-angiogenic therapy; Density; Immunotherapy; Melanoma; RECIST; Tumor response.
Figures

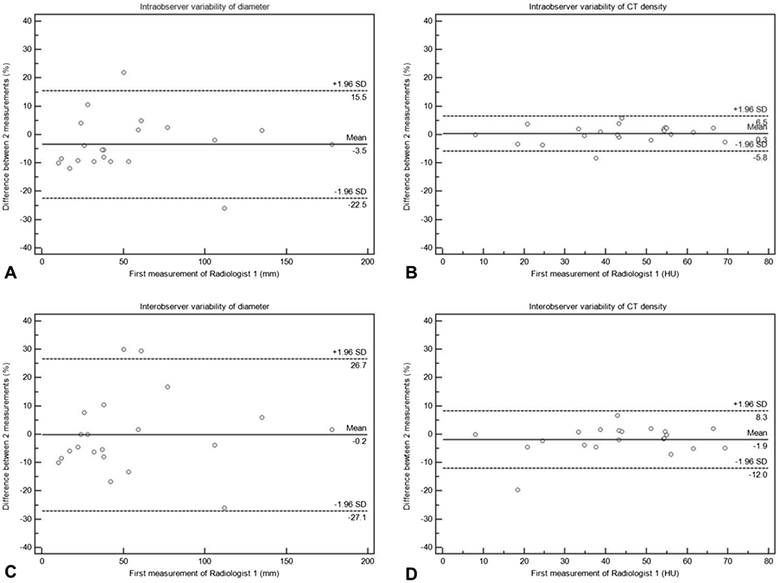
References
-
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic t lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. - DOI - PMC - PubMed
-
- Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N, Day AL, Kruse A, Mac Rae S, Hoos A, Mihm M. Ctla-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the cns. Nat Clin Pract Oncol. 2008;5:557–561. doi: 10.1038/ncponc1183. - DOI - PubMed
-
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
-
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage iiib/iv non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase ii study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. - DOI - PubMed
-
- Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
