Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms
- PMID: 25411968
- PMCID: PMC4239021
- DOI: 10.1371/journal.pone.0113054
Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms
Abstract
Background: Kawasaki disease (KD) is a systemic vasculitis of unknown etiology. The innate immune system is involved in its pathophysiology at the acute phase. We have recently established a novel murine model of KD coronary arteritis by oral administration of a synthetic microbe-associated molecular pattern (MAMP). On the hypothesis that specific MAMPs exist in KD sera, we have searched them to identify KD-specific molecules and to assess the pathogenesis.
Methods: We performed liquid chromatography-mass spectrometry (LC-MS) analysis of fractionated serum samples from 117 patients with KD and 106 controls. Microbiological and LC-MS evaluation of biofilm samples were also performed.
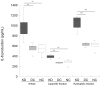
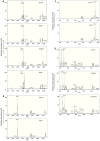
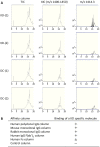
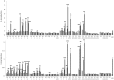
Results: KD samples elicited proinflammatory cytokine responses from human coronary artery endothelial cells (HCAECs). By LC-MS analysis of KD serum samples collected at 3 different periods, we detected a variety of KD-specific molecules in the lipophilic fractions that showed distinct m/z and MS/MS fragmentation patterns in each cluster. Serum KD-specific molecules showed m/z and MS/MS fragmentation patterns almost identical to those of MAMPs obtained from the biofilms formed in vitro (common MAMPs from Bacillus cereus, Yersinia pseudotuberculosis and Staphylococcus aureus) at the 1st study period, and from the biofilms formed in vivo (common MAMPs from Bacillus cereus, Bacillus subtilis/Bacillus cereus/Yersinia pseudotuberculosis and Staphylococcus aureus) at the 2nd and 3rd periods. The biofilm extracts from Bacillus cereus, Bacillus subtilis, Yersinia pseudotuberculosis and Staphylococcus aureus also induced proinflammatory cytokines by HCAECs. By the experiments with IgG affinity chromatography, some of these serum KD-specific molecules bound to IgG.
Conclusions: We herein conclude that serum KD-specific molecules were mostly derived from biofilms and possessed molecular structures common to MAMPs from Bacillus cereus, Bacillus subtilis, Yersinia pseudotuberculosis and Staphylococcus aureus. Discovery of these KD-specific molecules might offer novel insight into the diagnosis and management of KD as well as its pathogenesis.
Conflict of interest statement
Figures


References
-
- Newburger JW, Takahashi M, Gerber MA, Gewits MH, Tani LY, et al. (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110: 2747–2771. - PubMed
-
- Burns JC (2007) The riddle of Kawasaki disease. N Engl J Med 356: 659–661. - PubMed
-
- Foell D, Ichida F, Vogl T, Yu X, Chen R, et al. (2003) S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet 361: 1270–1272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

