Increased C-C chemokine receptor 2 gene expression in monocytes of severe obstructive sleep apnea patients and under intermittent hypoxia
- PMID: 25411969
- PMCID: PMC4239065
- DOI: 10.1371/journal.pone.0113304
Increased C-C chemokine receptor 2 gene expression in monocytes of severe obstructive sleep apnea patients and under intermittent hypoxia
Abstract
Background: Obstructive sleep apnea (OSA) is known to be a risk factor of coronary artery disease. The chemotaxis and adhesion of monocytes to the endothelium in the early atherosclerosis is important. This study aimed to investigate the effect of intermittent hypoxia, the hallmark of OSA, on the chemotaxis and adhesion of monocytes.
Methods: Peripheral blood was sampled from 54 adults enrolled for suspected OSA. RNA was prepared from the isolated monocytes for the analysis of C-C chemokine receptor 2 (CCR2). The effect of intermittent hypoxia on the regulation and function of CCR2 was investigated on THP-1 monocytic cells and monocytes. The mRNA and protein expression levels were investigated by RT/real-time PCR and western blot analysis, respectively. Transwell filter migration assay and cell adhesion assay were performed to study the chemotaxis and adhesion of monocytes.
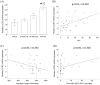
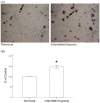
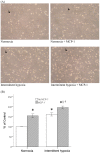
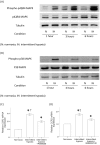
Results: Monocytic CCR2 gene expression was found to be increased in severe OSA patients and higher levels were detected after sleep. Intermittent hypoxia increased the CCR2 expression in THP-1 monocytic cells even in the presence of TNF-α and CRP. Intermittent hypoxia also promoted the MCP-1-mediated chemotaxis and adhesion of monocytes to endothelial cells. Furthermore, inhibitor for p42/44 MAPK or p38 MAPK suppressed the activation of monocytic CCR2 expression by intermittent hypoxia.
Conclusions: This is the first study to demonstrate the increase of CCR2 gene expression in monocytes of severe OSA patients. Monocytic CCR2 gene expression can be induced under intermittent hypoxia which contributes to the chemotaxis and adhesion of monocytes.
Conflict of interest statement
Figures


Similar articles
-
Monocytic C-C chemokine receptor 5 expression increases in in vitro intermittent hypoxia condition and in severe obstructive sleep apnea patients.Sleep Breath. 2019 Dec;23(4):1177-1186. doi: 10.1007/s11325-019-01797-4. Epub 2019 Feb 18. Sleep Breath. 2019. PMID: 30778913 Free PMC article.
-
Increased MCP-1 gene expression in monocytes of severe OSA patients and under intermittent hypoxia.Sleep Breath. 2016 Mar;20(1):425-33. doi: 10.1007/s11325-015-1252-5. Epub 2015 Sep 9. Sleep Breath. 2016. PMID: 26354107
-
Cilostazol reduces MCP-1-induced chemotaxis and adhesion of THP-1 monocytes by inhibiting CCR2 gene expression.Biochem Biophys Res Commun. 2011 Jul 29;411(2):402-8. doi: 10.1016/j.bbrc.2011.06.163. Epub 2011 Jul 2. Biochem Biophys Res Commun. 2011. PMID: 21756880
-
Leukotrienes as a molecular link between obstructive sleep apnoea and atherosclerosis.Cardiovasc Res. 2014 Feb 1;101(2):187-93. doi: 10.1093/cvr/cvt247. Epub 2013 Nov 7. Cardiovasc Res. 2014. PMID: 24204032 Review.
-
Obstructive sleep apnea and cancer: effects of intermittent hypoxia?Future Oncol. 2015;11(24):3285-98. doi: 10.2217/fon.15.216. Epub 2015 Nov 12. Future Oncol. 2015. PMID: 26562000 Review.
Cited by
-
Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models.Front Endocrinol (Lausanne). 2020 Feb 21;11:57. doi: 10.3389/fendo.2020.00057. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32153502 Free PMC article. Review.
-
Elevated Monocytic Interleukin-8 Expression under Intermittent Hypoxia Condition and in Obstructive Sleep Apnea Patients.Int J Mol Sci. 2021 Oct 22;22(21):11396. doi: 10.3390/ijms222111396. Int J Mol Sci. 2021. PMID: 34768826 Free PMC article.
-
C-C Chemokine Receptor Type 2 Expression on Monocytes Before Sepsis Onset Is Higher Than That of Postsepsis in Septic Burned Patients: A New Predictor for Sepsis in Burned Injury.Ann Surg. 2016 Aug;264(2):392-8. doi: 10.1097/SLA.0000000000001531. Ann Surg. 2016. PMID: 26727083 Free PMC article.
-
Engagement of monocytes, NK cells, and CD4+ Th1 cells by ALVAC-SIV vaccination results in a decreased risk of SIVmac251 vaginal acquisition.PLoS Pathog. 2020 Mar 12;16(3):e1008377. doi: 10.1371/journal.ppat.1008377. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32163525 Free PMC article.
-
Hypoxia Alters the Expression of CC Chemokines and CC Chemokine Receptors in a Tumor-A Literature Review.Int J Mol Sci. 2020 Aug 6;21(16):5647. doi: 10.3390/ijms21165647. Int J Mol Sci. 2020. PMID: 32781743 Free PMC article. Review.
References
-
- Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. American journal of respiratory and critical care medicine 165: 1217–1239. - PubMed
-
- Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. American Thoracic Society. Official statement adopted March 1944. Am J Respir Crit Care Med 150: 1738–1745. - PubMed
-
- Malhotra A, White DP (2002) Obstructive sleep apnoea. Lancet 360: 237–245. - PubMed
-
- Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, et al. (2001) Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 154: 50–59. - PubMed
-
- Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, et al. (1998) Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 97: 2154–2159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

