Biomechanics and mechanobiology of trabecular bone: a review
- PMID: 25412137
- PMCID: PMC5101038
- DOI: 10.1115/1.4029176
Biomechanics and mechanobiology of trabecular bone: a review
Abstract
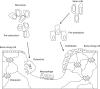
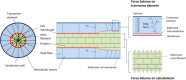
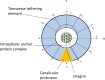
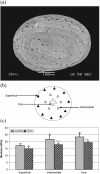
Trabecular bone is a highly porous, heterogeneous, and anisotropic material which can be found at the epiphyses of long bones and in the vertebral bodies. Studying the mechanical properties of trabecular bone is important, since trabecular bone is the main load bearing bone in vertebral bodies and also transfers the load from joints to the compact bone of the cortex of long bones. This review article highlights the high dependency of the mechanical properties of trabecular bone on species, age, anatomic site, loading direction, and size of the sample under consideration. In recent years, high resolution micro finite element methods have been extensively used to specifically address the mechanical properties of the trabecular bone and provide unique tools to interpret and model the mechanical testing experiments. The aims of the current work are to first review the mechanobiology of trabecular bone and then present classical and new approaches for modeling and analyzing the trabecular bone microstructure and macrostructure and corresponding mechanical properties such as elastic properties and strength.
Figures




References
-
- Vanderoost, J. , Jaecques, S. V. , Van der Perre, G. , Boonen, S. , D'hooge, J. , Lauriks, W. , and van Lenthe, G. H. , 2011, “Fast and Accurate Specimen-Specific Simulation of Trabecular Bone Elastic Modulus Using Novel Beam—Shell Finite Element Models,” J. Biomech., 44(8), pp. 1566–1572.10.1016/j.jbiomech.2011.02.082 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

