Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model
- PMID: 25412185
- PMCID: PMC4238990
- DOI: 10.1371/journal.pntd.0003295
Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model
Abstract
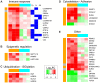
Infection with yellow fever virus (YFV), an explosively replicating flavivirus, results in viral hemorrhagic disease characterized by cardiovascular shock and multi-organ failure. Unvaccinated populations experience 20 to 50% fatality. Few studies have examined the pathophysiological changes that occur in humans during YFV infection due to the sporadic nature and remote locations of outbreaks. Rhesus macaques are highly susceptible to YFV infection, providing a robust animal model to investigate host-pathogen interactions. In this study, we characterized disease progression as well as alterations in immune system homeostasis, cytokine production and gene expression in rhesus macaques infected with the virulent YFV strain DakH1279 (YFV-DakH1279). Following infection, YFV-DakH1279 replicated to high titers resulting in viscerotropic disease with ∼72% mortality. Data presented in this manuscript demonstrate for the first time that lethal YFV infection results in profound lymphopenia that precedes the hallmark changes in liver enzymes and that although tissue damage was noted in liver, kidneys, and lymphoid tissues, viral antigen was only detected in the liver. These observations suggest that additional tissue damage could be due to indirect effects of viral replication. Indeed, circulating levels of several cytokines peaked shortly before euthanasia. Our study also includes the first description of YFV-DakH1279-induced changes in gene expression within peripheral blood mononuclear cells 3 days post-infection prior to any clinical signs. These data show that infection with wild type YFV-DakH1279 or live-attenuated vaccine strain YFV-17D, resulted in 765 and 46 differentially expressed genes (DEGs), respectively. DEGs detected after YFV-17D infection were mostly associated with innate immunity, whereas YFV-DakH1279 infection resulted in dysregulation of genes associated with the development of immune response, ion metabolism, and apoptosis. Therefore, WT-YFV infection is associated with significant changes in gene expression that are detectable before the onset of clinical symptoms and may influence disease progression and outcome of infection.
Conflict of interest statement
Oregon Health and Science University, MKS, and EH declare a financial interest based on shares in Najít Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by Oregon Health and Science University. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures




References
-
- Russell MN, Cetron MS, Eidex RB (2006) The US-Certified Yellow Fever Vaccination Center Registry: a tool for travelers, state health departments, and vaccine providers. J Travel Med 13: 48–49. - PubMed
-
- Tomori O (2004) Yellow fever: the recurring plague. Crit Rev Clin Lab Sci 41: 391–427. - PubMed
-
- Barrett AD, Higgs S (2007) Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol 52: 209–229. - PubMed
-
- Paessler S, Walker DH (2013) Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol 8: 411–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

