Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders
- PMID: 25412207
- PMCID: PMC4238950
- DOI: 10.1371/journal.pcbi.1003956
Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders
Abstract
Misfolded proteins (MP) are a key component in aging and associated neurodegenerative disorders. For example, misfolded Amyloid-ß (Aß) and tau proteins are two neuropathogenic hallmarks of Alzheimer's disease. Mechanisms underlying intra-brain MP propagation/deposition remain essentially uncharacterized. Here, is introduced an epidemic spreading model (ESM) for MP dynamics that considers propagation-like interactions between MP agents and the brain's clearance response across the structural connectome. The ESM reproduces advanced Aß deposition patterns in the human brain (explaining 46∼56% of the variance in regional Aß loads, in 733 subjects from the ADNI database). Furthermore, this model strongly supports a) the leading role of Aß clearance deficiency and early Aß onset age during Alzheimer's disease progression, b) that effective anatomical distance from Aß outbreak region explains regional Aß arrival time and Aß deposition likelihood, c) the multi-factorial impact of APOE e4 genotype, gender and educational level on lifetime intra-brain Aß propagation, and d) the modulatory impact of Aß propagation history on tau proteins concentrations, supporting the hypothesis of an interrelated pathway between Aß pathophysiology and tauopathy. To our knowledge, the ESM is the first computational model highlighting the direct link between structural brain networks, production/clearance of pathogenic proteins and associated intercellular transfer mechanisms, individual genetic/demographic properties and clinical states in health and disease. In sum, the proposed ESM constitutes a promising framework to clarify intra-brain region to region transference mechanisms associated with aging and neurodegenerative disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

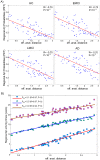
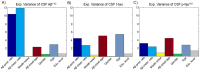
 . Then, a selective iterative algorithm estimated, for each subject, the model parameters that maximized the similarity between the generated and the reference Aß deposition pattern, as well as the time point at which this maximization occurred. The latter output was used to calculate the individual onset age of Aß binding, which in conjunction with the obtained model parameters were assumed to characterize each subject's Aß propagation/deposition history.
. Then, a selective iterative algorithm estimated, for each subject, the model parameters that maximized the similarity between the generated and the reference Aß deposition pattern, as well as the time point at which this maximization occurred. The latter output was used to calculate the individual onset age of Aß binding, which in conjunction with the obtained model parameters were assumed to characterize each subject's Aß propagation/deposition history.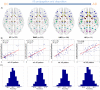


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

